Abstract
Industrial wastewater that contains trace amounts of heavy metal ions is often seen in petrochemical industry. While this wastewater can not be directly discharged, it is difficult to treat due to the low concentration of metal ions. Introducing chelating reagents into this wastewater for selective ion adsorption, followed by a mechanical separation process, provides an appealing solution. Toward the success of this technology, the development of effective chelating resins is of key importance. In the present work, a chelating resin containing amino and dithiocarbamate groups was reported for the removal of Co(II) metal ions in trace concentrations from simulated wastewater. By investigating the adsorption performance of the chelating resin at different solution pH values, adsorbent dosages, contact time, initial ion concentrations, and adsorption temperatures, the maximum adsorption capacity of the resin for Co(II) was identified to be 24.89 mg g−1 for a 2 g L−1 adsorbent dosage and a pH value of 5. After four adsorption–desorption cycles, 97% of the adsorption capacity of the resin was maintained. The adsorption kinetics and thermodynamics were analyzed and discussed as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wastewater with heavy metal ions has aroused great concern because of their general and specific toxic nature and other adverse effects on human life. To address this concern, various methods, such as chemical precipitation adsorption (Liu et al. 2015; Huisman et al. 2006; Özverdi and Erdem 2006), adsorption (Cui et al. 2014a, b; Kang et al. 2008), ionic exchange (Zhang et al. 2015; Li et al. 2014; Xie et al. 2014; Gode and Pehlivan 2006), electrochemical treatment (Chen 2004; Heidmann and Calmano 2008; Nanseu-Njiki et al. 2009), coagulation and flocculation (El Samrani et al. 2008), flotation (Lundh et al. 2000; Yuan et al. 2008), and membrane separation technique (Du et al. 2014), have been proposed for the development of cheap and effective wastewater treatment technologies. While each method has its own advantage for treating wastewater with different levels of metal ion concentrations, adsorption is the commonly used one and it has been widely employed in industry to remove hazardous metal ions or other contaminants from wastewater. This is probably because the adsorption process is cheap to operate and flexible to maintain, and it also generates a high quality effluent, even when metal ions are in trace concentrations in the feed wastewater (Fu and Wang 2011). Thus, many inorganic and organic adsorbents have been prepared or examined, including zeolites (Jovanovic et al. 2012; Padervand and Gholami 2013), clay minerals (Al-Jlil and Alsewailem 2009), fly ash (Gupta and Torres 1998), biosorbents (Kadirvelu et al. 2001), and activated carbon (Machida et al. 2012). Generally, these adsorbents exhibit high adsorption capacity, but limitations such as high operational costs, low recyclability and the introduction of a large volume of additional contaminants, have also been found. Therefore, intensive studies have been carried out to develop more efficient, environmental friendly and inexpensive adsorbents for the removal of heavy metal ions from wastewater. Kongsuwan et al. (Kongsuwan et al. 2009) explored the use of activated carbon from eucalyptus bark in a binary component adsorption of Cu(II) and Pb(II), and the maximum adsorption capacities achieved were 0.45 mmol g−1 for Cu(II) and 0.53 mmol g−1 for Pb(II). Agoubordea and Navia (Agouborde and Navia 2009) investigated brine sediments, sawdust and the mixture of both materials to remove Zn(II) or Cu(II) from aqueous solutions; the maximum adsorption capacities of these three adsorbents were identified to be 4.85, 2.58 and 5.59 mg g−1 for Zn(II) and 4.69, 2.31 and 4.33 mg g−1 for Cu(II), respectively. Gao et al. (Gao et al. 2009) investigated anion removal from aqueous solutions using grafting particles PEI@SiO2, and a high saturated adsorption amount for CrO4 2− ions of up to 0.14 g g−1 was reported, owing to electrostatic interactions.
The removal of Co(II) from industrial wastewater is demanded in petrochemical plants, where Co(II) comes primarily from the utilization of metal catalysts for the production of chemical raw materials such as pure terephthalic acid. The concentration of Co(II) in initial wastewater is readily high up to 2.0 g L−1. After preliminary treatment with sedimentation, the ion concentration can be reduced by up to 90%. However, the resultant concentration of Co(II) is still much beyond the effluent standard in China, namely 1.0 mg L−1 (National Standards of Integrated Wastewater Discharge Standard, People’s Republic of China 1996). The pre-treated wastewater is usually recycled by mixing with fresh water for the purpose of energy-saving and emission reduction. Nevertheless, the recycling of wastewater that contains even trace amounts of heavy metal ions causes several troublesome problems. First, the deposition of heavy metal ions in biochemical ponds gradually reduces the biological activity of bacteria, and thereafter depresses the degradation efficiency of organic contaminants (Máthé et al. 2012). Second, charged metal ions have a strong tendency to associate with many membranes utilized for filtration, causing membrane fouling (Zhao et al. 2016). Third, the presence of metal ions in feed wastewater can result in serious corrosion of metal devices through an ion-exchange process. Whereas it is relatively easy to reduce the concentration of heavy metal ions from a high value to a low one, the removal of trace amounts of heavy metal ions from wastewater is much more challenging. Introducing chelating reagents for ion adsorption, followed by a mechanical separation process, provides one promising solution. The realization of this method highly relies on the development of excellent chelating reagents.
In this work, a dithiocarbamate (DTC) resin prepared as a novel adsorbent for the removal of Co(II) from simulated wastewater was presented. DTC molecular group can strongly bind with various heavy metal ions and display high removal efficiency due to its strong tendency of sharing electrons between N, S elements and heavy metal ions (Bai et al. 2011). For example, Fan et al. (2014a, b, c) prepared three different silica-supported ion-imprinted hybrid sorbents all functionalized with S, N-donor atoms for lead (II), cadmium (II) and lead (II) through the surface imprinting technique. The sorbents showed high adsorption for Pb(II) (54.9 mg g−1), Cd(II) (29.1 mg g−1) and Pb(II) (61.9 mg g−1) within 30 min, respectively. In addition, Fan et al. (Fan et al. 2011) presented a Co(II)-imprinted silica gel sorbent with the same technique as mentioned above and the maximum adsorption capacities toward Co(II) was 35.2 mg g−1 compared to 6.5 mg g−1 which is the non-imprinted. Hence, micromolecular DTC is usually synthesized from carbon disulfide and amino compounds, including diethylenetriamine, triethylenetetramine, ethylenediamine, diethylamine, etc., under an alkaline environment (Stathi et al. 2010; Jones et al. 1980; Shaaban et al. 2013; Horvath and Barnes 1986). However, many limitations have also been found, such as being volatilizable, very expensive, not easily available, and toxic (Talebi et al. 2010; Ling et al. 2011). To tackle these problems, the DTC resin here was developed by grafting polyethyleneimine (PEI) onto chloromethylated polystyrene (CPS) beads (Gao et al. 2006) as the amino compound. To examine the adsorption capability, different simulated wastewater bodies with various Co(II) concentrations in the range of 40–320 mg L−1 were tested. The maximum adsorption conditions of Co(II) have been identified and the nature of the adsorption process with respect to its kinetics and thermodynamic aspects were investigated.
Experimental
Materials and instruments
Chloromethylated polystyrene beads were purchased from the Chemical Plant of Nan Kai University (Tianjin, China), and polyethyleneimine was purchased from Sigma Aldrich Co. (USA). Carbon disulfide, sodium hydroxide, chloroform, and alcohol were purchased from General Chemical (Shanghai, China). All other reagents were of analytical grade and used as received. All solutions were prepared with ultrapure water.
In the ion adsorption experiments, the pH values of the aqueous solutions were measured using a Hanna HI221 model pH meter, and the temperature-dependent experiments were carried out in a Nickel Electro Clifton NE1-22 model thermostatic bath. The concentration of Co(II) ions was measured using a Perkin-Elmer Analyst 200 model atomic absorption spectrophotometer (AAs).
Synthesis and characterization
The synthesis procedure of the chelating resin involved three steps:
-
(i)
10.0 g of the CPS beads was added into 50 ml of chloroform in a round-bottom flask, and the resultant suspension was stirred at 600 rpm at 25 °C for 12 h. Afterward, the swollen CPS beads were washed with alcohol and ultrapure water three times and then separated from the supernatant by filtration;
-
(ii)
4.0 g of sodium hydroxide was dissolved in 100 ml of ultrawater, and 10 g of PEI was added to this solution. Then, the pretreated CPS beads were added to the solution and the mixture was stirred at 600 rpm at 50 °C for 15 h;
-
(iii)
The pretreated CPS beads carrying the PEI were added into a mixture of 100 ml of ultrawater, 10 ml of carbon disulfide and 4 g of sodium hydroxide. The resultant mixture was stirred at 600 rpm at 20 °C for 12 h, continuously at 50 °C for another 12 h. The obtained product was washed individually with water and ethanol; then, it was dried in a vacuum oven at room temperature, milled, and sieved for utilization in the adsorption experiments.
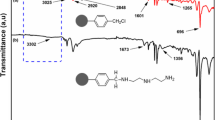
Figure 1 showed the FT-IR spectra of DTC chelating resin. The adsorption peaks at 1617 and 1300 cm−1 stand for C=N and C–N stretching vibrations. The peak at 3363 cm−1 is caused by the N–H stretching vibrations, and the characteristic peak at 1071 cm−1 is attributed to C=S stretching vibrations, indicating that DTC functional group is attached to the resin.
Adsorption studies
All of the adsorption experiments, including pH effect, adsorbent dosage, kinetic and isotherm study, were conducted batch-wise.
A 320 mg L−1 (320 ppm) stock solution of Co(II) was prepared by dissolving CoCl2·6H2O in ultrapure water. Solutions with different Co(II) concentrations in a range of 40–320 mg L−1 were prepared by diluting the stock solution. Adsorption experiments related to pH and adsorbent dosage effect were performed at 25 °C in the thermostatic bath. To determine the effect of pH value, adsorption experiments were carried out at different pHs in a range from 1 to 5 by mixing 100 ml of 80 mg L−1 aqueous Co(II) solutions with 0.2 g of adsorbent. The pH values of the initial solutions were adjusted by adding dilute NaOH or HCl solutions. To assess the optimal adsorbent dosage, 100 ml 80 mg L−1 aqueous Co(II) solutions were mixed with varying amounts of adsorbent in a range of 0.2–1.0 g at pH 5.
The adsorption isotherms were evaluated through a series of adsorption measurements at three different temperatures (20, 30 and 40 °C) and different initial Co(II) ion concentrations in the range of 40–320 mg L−1 at pH 5. To achieve adsorption equilibrium, the adsorption time was extended to 24 h in these experiments. The Co(II) ion concentration was monitored using an atomic absorption spectrophotometer in the adsorption experiments, and then the adsorption capacity, i.e., \(q_{\text{e}}\) (mg g−1), of the adsorbent was calculated as
where \(C_{0}\) and \(C_{\text{e}}\) are, respectively, the initial and final concentrations of Co(II) ions in the solution (mg L−1); \(V\) is the volume of the solution (L) and \(m\) is the weight of the adsorbent (g). All assays were carried out in triplicate and only the average values were presented. The removal efficiency (Re %) of the resin was calculated using the following conventional equation:
The kinetic studies were carried out at 25 °C, and the initial metal ion concentration, adsorbent dosage and the pH of the adsorption solutions were chosen to be 80 mg L−1, 0.2 g/100 ml, and 5, respectively.
Desorption and regeneration assay
The desorption of metal ions adsorbed on the resin was performed by the agitated mixing of 0.2 g of Co(II)-loaded resins and 20 mL of 0.1 mol L−1 HCl solution at room temperature for 4 h. After filtration, the final metal ion concentrations in the aqueous solution were determined by AAs. The desorption ratio (De %) was calculated by dividing the amount of heavy metal ion desorbed into the acid medium by the total amount adsorbed onto the DTC-chelating resin.
After the desorption process, the collected DTC resin was washed thoroughly three times with ultrapure water and then dried in a vacuum for the next adsorption test. To access the reusability of the DTC resin, the adsorption–desorption cycles were repeated four times by using the same affinity adsorbent.
Figure 2a–c displayed the simulated wastewater after 0, 60 and 120 min with the introduction of DTC resin, and the temperature, initial metal ion concentration, adsorbent dosage and the pH of the adsorption solutions were 25 °C, 80 mg L−1, 0.2 g/100 ml, and 5, respectively. Apparently, the adsorption of Co(II) ion has bleached the color of simulated wastewater. Figure 2d–f presented the DTC resins before and after the usage of wastewater treatment. It was seen that the yellow DTC resins became green after adsorbing Co(II) ions.
Results and discussions
Effects of pH and adsorbent dosage
It has been reported that the pH value alters the metal chemistry in a solution or the protonation or deprotonation of the adsorbents (Bayramoglu et al. 2002). Figure 3 showed the effects of pH and dosage on the adsorption of Co(II). First, it was clearly seen that the affinity of resin towards Co(II) was sensitive to the pH value. When the pH decreased, the adsorption capacity became smaller. This is likely due to the competitive adsorption of H+ ions with Co(II) on the same active site. This finding coincides with previous studies (Liu et al. 2006; An et al. 2011). When the pH value was higher than 5, Co(II) might have the hydrolysis reaction and lead up to the hydroxyl complexes, resulting in a lower adsorption capacity. Because the pH of industrial wastewater is approximately 5, we adopted the simulated wastewater with pH = 5 in the following studies (unless it was specified otherwise). Second, the curve of the adsorption capacity as a function of the adsorbent dosage showed that, when more dosage was added, the adsorption capacity decreased; meanwhile, the removal efficiency increased, indicating that a tradeoff between adsorption capability and removal efficiency should be considered in practical usage.
Adsorption kinetics
Contact time is a crucial parameter directly associated with the design and operation of a separation technology. Figure 4 showed the adsorption capacity of Co(II) as a function of contact time onto the DTC resin. During the initial 1-h adsorption period, the adsorption capacity, \(q_{\text{e}}\), presented a sharp increase with increasing contact time, reaching a plateau after almost 3 h. Specifically, within the first hour, \(q_{\text{e}}\) reached 19.87 mg g−1 (79.8% of the total adsorption); at 3 h, more than 97% of the total adsorption was completed, and \(q_{\text{e}}\) was found to be 24.14 mg g−1. After 3 h, the adsorption rate slowed down and, after 5 h, reached equilibrium, while the maximum of \(q_{\text{e}}\) was found to be 24.89 mg g−1.
Table 1 presented the maximum adsorption capacity of Co(II) ion in different adsorbents. Apparently, the adsorption capacity of DTC resin was very competitive.
To clarify the kinetic characteristics of the adsorption, different kinetic models were adopted to analyze the experimental data in this work. We found that the adsorption kinetics could be well described with the pseudo-second-order kinetic model, which is given by the equation (Ho and McKay 1999).
where \(K\) (g mg−1 min−1) is the rate constant of the pseudo-second-order adsorption reaction. A second-order kinetic can be applicable if the plot of \(t/q_{t}\) versus \(t\) shows linearity. Figure 5 showed the plots obtained from the graphical interpretation of the data for the second-order kinetic model, as well as the corresponding fitting parameters K = 0.00268 and \(q_{\text{e}}\) = 25.3 mg g−1.
In addition to the very high correlation coefficient of the fitting curve (\(R^{2} = 0.9998\)), the excellent accordance between experimental (24.89 mg g−1) and theoretical (25.3 mg g−1) \(q_{\text{e}}\) values confirms that the adsorption of Co(II) metal ions onto the DTC resin follows pseudo-second-order type reaction kinetics. It is generally understood that pseudo-second-order kinetics provide the best fits to the experimental data for the adsorption systems where chemisorption seems significant in the rate controlling step (Ho and McKay 1999; Zheng and Wang 2010); therefore, for our DTC resin, the chemisorption should be the rate determining step in the adsorption process.
Adsorption isotherm
The removal of Co(II) by the DTC resin as a function of the initial concentration was studied at three typical temperatures (20, 30, 40 °C) while varying the ion concentration from 40 to 320 mg L−1, keeping all other parameters constant. The results were displayed in Fig. 6. It was shown that \(q_{\text{e}}\) values tended to increase significantly with the increase of the initial Co(II) concentration. In addition, a higher temperature results in a bigger \(q_{\text{e}}\), indicating that the adsorption of Co(II) onto the resin is an endothermic process.
There are two most generally used isotherms, namely Langmuir and Freundlich adsorption isotherms. We found that the adsorption isotherm could be analyzed with the Langmuir adsorption model. The linear form of the Langmuir equation is given as (Langmuir 1918).
The linear form of the Freundlich equation is given as (Freundlich 1906).
where \(q_{\hbox{max} }\) is the monolayer capacity of the adsorbent (mol g−1) and \(b\) is the Langmuir constant (L mol−1) related to the free energy of adsorption; K f is the Freundlich constant and n is the indicator of adsorption intensity. The plots of Langmuir adsorption at the three temperatures are shown in Fig. 7. The parameters and correlation coefficients obtained from these plots are listed in Table 2. For all temperatures, the Langmuir correlation coefficients are closer to 1 than Freundlich, suggesting that Co(II) adsorption on the adsorbent can be interpreted using the Langmuir adsorption model.
The adsorption capacity of an adsorbent was represented as \(q_{\hbox{max} }\). As seen from Table 2, \(q_{\hbox{max} }\) increased with increasing temperature, indicating the endothermic nature of the adsorption process. In addition, the favorability of the adsorption process can be determined by the separation constant, i.e., \(R_{\text{L}}\) (Namasivayam and Ranganathan 1993; Dalaran et al. 2009), which is defined as
For all initial metal ion concentrations in the range of 40–320 mg L−1, the \(R_{\text{L}}\) values were found to be between 0 and 1.0, which indicated that the adsorption of Co(II) onto the DTC resin was a favorable adsorption. The \(R_{\text{L}}\) values are listed in Table 2. It was clear that a higher temperature resulted in a higher \(R_{\text{L}}\) value, showing that adsorption was more favorable at lower temperatures.
The adsorption mechanism can be suggested by the mean energy of adsorption; the latter refers to the free energy change when transferring one mole of adsorbate from the bulk solution to the surface of the adsorbent, and it can be calculated (Hobson 1969) as \(E_{\rm a} = 1/\sqrt {2\beta }\). Here, \(\beta\) (mol2 kJ−2) is the constant in the Dubinin–Radushkevich (D-R) (1947) isotherm equation
where \(q_{\text{m}}\) is the theoretical saturation capacity; \(\varepsilon\) is the Polanyi potential, which is equal to \(R_{\text{c}} T\ln (1 + 1/C_{\text{e}} )\), with \(R_{\text{c}}\) (J mol−1 K−1) being the gas constant and \(T\) (K) the absolute temperature. The two parameters \(q_{\text{m}}\) and \(\beta\) can be obtained by fitting the experimental adsorption isotherm curves in Fig. 5. Both parameters, together with the thereafter calculated mean energies of adsorption, are presented in Table 2.
It is said that when the value of \(E_{\rm a}\) is in the range of 1–8 kJ mol−1, it corresponds to a process dominated by physical adsorption; in the range of 8–16 kJ mol−1 (Onyango et al. 2004; Helfferich 1962), it corresponds to a process dominated by an ion-exchange mechanism. In Table 2, \(E_{\rm a}\) values were higher than 16 kJ mol−1 at 293.15 and 303.15 K, which indicates that neither physical adsorption nor an ion-exchange mechanism is the dominant factor, and another mechanism with an even stronger effect on the adsorption should be accounted for. Considering the DTC and amino functional groups on the resin surface, the adsorption of Co(II) occurs through electrostatic attraction, ion-exchange, and chelating mechanisms, simultaneously. Thus, by excluding the physical adsorption and ion-exchange mechanisms as the dominating factors, it is safe to deduce that the chelating mechanism has a dominant effect on Co(II) adsorption on the resin. However, the value of \(E_{\rm a}\) at 313.15 K dropped into the range of 8–16 kJ mol−1, indicating that the ion-exchange mechanism takes over the dominant position. This is because, at high temperatures, ion-exchange becomes relatively more favorable than chelating.
Adsorption thermodynamics
Thermodynamic parameters, including changes in the Gibbs free energy (\(\Delta G^{0}\)), enthalpy (\(\Delta H^{0}\)), and entropy (\(\Delta S^{0}\)), are the actual indicators for practical application of an adsorption process. According to values of these parameters, the spontaneously occurring process can be determined. The thermodynamic data were calculated from the Langmuir isotherms using the following equations:
where \(b_{1}\) and \(b_{2}\) are the Langmuir constants [or, equivalently, \(b\) in Eq. (7)]at temperatures \(T_{1}\) and \(T_{2}\), respectively. \(b\) at three different temperatures are listed in Table 2.
The thermodynamic properties for the present adsorption systems at three different temperatures are given in Table 3. As shown, the \(\Delta G^{0}\) value was negative, and it decreased slightly with increasing temperature, indicating the spontaneous nature of the adsorption process and a more spontaneous tendency at higher temperatures. Generally, the Gibbs free energy for a physisorption is in the range of −20 to 0 kJ mol−1; however, that for a chemisorption is much lower, in the range of −80 to −400 kJ mol−1 (Donia et al. 2006). The values of \(\Delta G^{0}\) in the present work were slightly lower than that of a physisorption, but much higher than that of a chemisorption, suggesting that both the physisorption and chemisorption mechanisms occur in the adsorption process. Thus, a mechanism combining chelation and ion exchange took place during the process, coinciding with the above kinetics analysis and isotherms models studies. Finally, a positive \(\Delta H^{0}\) suggests an endothermic reaction.
Desorption and regeneration study
The discussion of pH effect on the adsorption capacity suggested that desorption of the adsorbed cobalt ions on the chelating resin can be easily performed in an acidic solution. A similar study (Say et al. 2006) also reported the effectiveness of acidic conditions on the desorption of adsorbed metals. Therefore, the desorption was performed in a 0.1 mol L−1 HCl solution. As seen in Table 4, the chelating resin had its largest initial adsorption capacity during the first cycle. The De % of the chelating resin was found to be 99.1% after the first cycle and then gradually decreased to 98.7, 98.1 and 97.3% for the second, third and fourth cycles, respectively. It was observed that the Co(II) adsorption capacity of the resin did not show a significant loss for each repeated adsorption–desorption cycle, and the adsorption capacity of the resin for the fourth adsorption was found to be 24.60 mg g−1, which indicates approximately a 4% loss of adsorption capacity from the initial use. Thus, the chelating resins synthesized here have good regeneration capacity and can be reused without significant decreasing in the Co(II) adsorption capacity and the recover efficiency after several cycles.
Conclusions
In the present study, a novel DTC functionalized resin was synthesized, and its adsorption characteristics for Co(II) were investigated. Adsorption studies showed that the resin presented a high adsorption capacity. The adsorbent dosage and pH effect studies showed that a 2 g L−1 dosage and pH = 5 are the optimum conditions to attain the maximum adsorption capacity.
The adsorption processes of Co(II) onto the DTC resin were found to undergo three stages and follow pseudo-second-order type adsorption kinetics. The adsorption pattern on the composite seems to follow the Langmuir isotherm. According to the variations of the IR spectra and the mean free energies of adsorption (\(E_{\rm a}\)), it can be safely concluded that the adsorption process is controlled simultaneously by the ion-exchange and chelating mechanisms. The calculated values of different thermodynamic parameters clearly indicate that the ongoing adsorption process is feasible, spontaneous, and endothermic in nature. Desorption and reuse experiments showed that the dithiocarbamate resin could be used at least four times without any significant loss in the adsorption performance. All of these findings indicate that the dithiocarbamate resin can be used as an effective adsorbent for the removal of Co(II) from aqueous solutions.
References
Agouborde L, Navia R (2009) Heavy metals retention capacity of a non-conventional sorbent developed from a mixture of industrial and agricultural wastes. J Hazard Mater 167(1–3):536–544
Ahmadpour A, Tahmasbi M, Bastami TR et al (2009) Rapid removal of cobalt ion from aqueous solutions by almond green hull. J Hazard Mater 166(2–3):925–930
Al-Jlil SA, Alsewailem FD (2009) Saudi Arabian clays for lead removal in wastewater. Appl Clay Sci 42(3–4):671–674
An F, Gao B, Dai X et al (2011) Efficient removal of heavy metal ions from aqueous solution using salicylic acid type chelate adsorbent. J Hazard Mater 192(3):956–962
Bai L, Hu H, Fu W et al (2011) Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J Hazard Mater 195:261–275
Bayramoglu G, Denizli A, Bektas S et al (2002) Entrapment of Lentinus sajor-caju into Ca-alginate gel beads for removal of Cd(II) ions from aqueous solution: preparation and biosorption kinetics analysis. Microchem J 72(1):63–76
Bhatnagar A, Minocha AK, Sillanpää M (2010) Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem Eng J 48(2):181–186
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38(1):11–41
Cui L, Hu L, Guo X et al (2014a) Kinetic, isotherm and thermodynamic investigations of Cu2+ adsorption onto magnesium hydroxyapatite/ferroferric oxide nano-composites with easy magnetic separation assistance. J Mol Liq 198:157–163
Cui L, Xu W, Guo X et al (2014b) Synthesis of strontium hydroxyapatite embedding ferroferric oxide nano-composite and its application in Pb2+ adsorption. J Mol Liq 197:40–47
Dalaran M, Emik S, Güçlü G et al (2009) Removal of acidic dye from aqueous solutions using poly(DMAEMA–AMPS–HEMA) terpolymer/MMT nanocomposite hydrogels. Polym Bull 63(2):159–171
Donia AM, Atia AA, El-Boraey HA et al (2006) Adsorption of Ag(I) on glycidyl methacrylate/N, N′-methylene bis-acrylamide chelating resins with embedded iron oxide. Sep Purif Technol 48(3):281–287
Du X, Zhang H, Hao X et al (2014) Facile preparation of ion-imprinted composite film for selective electrochemical removal of nickel(II) ions. ACS Appl Mater Interfaces 6(12):9543–9549
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of activated charcoal. In: Proceedings of the academy of sciences. Physical Chemistry Section USSR, pp 331–333
Ebner AD, Ritter JA, Navratil JD (2001) Adsorption of cesium, strontium, and cobalt ions on magnetite and a magnetite-silica composite. Ind Eng Chem Res 40(7):1615–1623
El Samrani AG, Lartiges BS, Villiéras F (2008) Chemical coagulation of combined sewer overflow: heavy metal removal and treatment optimization. Water Res 42(4–5):951–960
Fan HT, Sui DP, Zhang LX et al (2011) Preparation of cobalt(II) ion imprinted silica gel sorbents by surface imprinting technique and its adsorption properties. Chem J Chin Univ 32(12):2902–2907
Fan HT, Liu MX, Na SB et al (2014a) Preparation, characterization, and selective adsorption for lead(II) of imprinted silica-supported organic–inorganic hybrid sorbent functionalized with chelating S, N-donor atoms. Monatshefte für Chemie Chem Mon 146(3):459–463
Fan HT, Liu JX, Yao H et al (2014b) Ionic imprinted silica-supported hybrid sorbent with an anchored chelating schiff base for selective removal of cadmium(II) ions from aqueous media. Ind Eng Chem Res 53(1):369–378
Fan HT, Sun XT, Zhang ZG et al (2014c) Selective removal of lead(II) from aqueous solution by an ion-imprinted silica sorbent functionalized with chelating N-donor atoms. J Chem Eng Data 59(6):2106–2114
Freundlich HMF (1906) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92(3):407–418
Gao B, Wang X, Shen Y (2006) Studies on characters of immobilizing penicillin G acylase on a novel composite support PEI/SiO2. Biochem Eng J 28(2):140–147
Gao B, Li Y, Chen Z (2009) Adsorption behaviour of functional grafting particles based on polyethyleneimine for chromate anions. Chem Eng J 150(2–3):337–343
Gode F, Pehlivan E (2006) Removal of chromium(III) from aqueous solutions using Lewatit S 100: the effect of pH, time, metal concentration and temperature. J Hazard Mater 136(2):330–337
Gupta G, Torres N (1998) Use of fly ash in reducing toxicity of and heavy metals in wastewater effluent. J Hazard Mater 57(1–3):243–248
Heidmann I, Calmano W (2008) Removal of Zn(II), Cu(II), Ni(II), Ag(I) and Cr(VI) present in aqueous solutions by aluminium electrocoagulation. J Hazard Mater 152(3):934–941
Helfferich F (1962) Ion exchange. McGraw-Hill Book Co., New York
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Hobson JP (1969) Physical adsorption isotherms extending from ultrahigh vacuum to vapor pressure. J Phys Chem 73(8):2720–2727
Horvath Z, Barnes RM (1986) Characterization of functional group complexation of a poly(dithiocarbamate) chelating resin. Anal Chem 58(4):725–727
Huisman JL, Schouten G, Schultz C (2006) Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 83(1–4):106–113
Jones MM, Burka LT, Hunter ME et al (1980) Dithiocarbamate chelating agents for toxic heavy metals. J Inorg Nucl Chem 42(5):775–778
Jovanovic M, Rajic N, Obradovic B (2012) Novel kinetic model of the removal of divalent heavy metal ions from aqueous solutions by natural clinoptilolite. J Hazard Mater 233–234:57–64
Kadirvelu K, Thamaraiselvi K, Namasivayam C (2001) Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Biores Technol 76(1):63–65
Kang KC, Kim SS, Choi JW et al (2008) Sorption of Cu2+ and Cd2+ onto acid- and base-pretreated granular activated carbon and activated carbon fiber samples. J Ind Eng Chem 14(1):131–135
Kara M, Yuzer H, Sabah E, Celik MS (2003) Adsorption of cobalt from aqueous solutions onto sepiolite. Water Res 37(1):224–232
Kongsuwan A, Patnukao P, Pavasant P (2009) Binary component sorption of Cu(II) and Pb(II) with activated carbon from Eucalyptus camaldulensis Dehn bark. J Ind Eng Chem 15(4):465–470
Langmuir I (1918) The adsorption of gases on plane SURFACES of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403
Li J-R, Wang X, Yuan B et al (2014) Layered chalcogenide for Cu2+ removal by ion-exchange from wastewater. J Mol Liq 200:205–212
Ling Y, Dai Y, Liu L (2011) Synthesis of a heavy metal chelating agent with DTC group and its performance of treatment of copper-containing wastewater. Chin J Environ Eng 5(6):1246
Liu C, Bai R, Hong L (2006) Diethylenetriamine-grafted poly(glycidyl methacrylate) adsorbent for effective copper ion adsorption. J Colloid Interface Sci 303(1):99–108
Liu Y, Zhao Y, Wang Y et al (2015) Polyamine-capped gold nanorod as a localized surface Plasmon resonance probe for rapid and sensitive copper(II) ion detection. J Colloid Interface Sci 439:7–11
Lundh M, Jönsson L, Dahlquist J (2000) Experimental studies of the fluid dynamics in the separation zone in dissolved air flotation. Water Res 34(1):21–30
Machida M, Fotoohi B, Amamo Y et al (2012) Cadmium(II) and lead(II) adsorption onto hetero-atom functional mesoporous silica and activated carbon. Appl Surf Sci 258(19):7389–7394
Máthé I, Benedek T, Táncsics A et al (2012) Diversity, activity, antibiotic and heavy metal resistance of bacteria from petroleum hydrocarbon contaminated soils located in Harghita County (Romania). Int Biodeterior Biodegradation 73:41–49
Namasivayam C, Ranganathan K (1993) Waste Fe(III)/Cr(III) hydroxide as adsorbent for the removal of Cr(VI) from aqueous solution and chromium plating industry wastewater. Environ Pollut 82(3):255–261
Nanseu-Njiki CP, Tchamango SR, Ngom PC et al (2009) Mercury(II) removal from water by electrocoagulation using aluminium and iron electrodes. J Hazard Mater 168(2–3):1430–1436
National Standards of Integrated Wastewater Discharge Standard, People’s Republic of China. 1996
Onyango MS, Kojima Y, Aoyi O et al (2004) Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J Colloid Interface Sci 279(2):341–350
Özverdi A, Erdem M (2006) Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J Hazard Mater 137(1):626–632
Padervand M, Gholami M (2013) Removal of toxic heavy metal ions from waste water by functionalized magnetic core–zeolitic shell nanocomposites as adsorbents. Environ Sci Pollut Res 20(6):3900–3909
Say R, Birlik E, Denizli A et al (2006) Removal of heavy metal ions by dithiocarbamate-anchored polymer/organosmectite composites. Appl Clay Sci 31(3–4):298–305
Shaaban AF, Fadel DA, Mahmoud AA et al (2013) Synthesis and characterization of dithiocarbamate chelating resin and its adsorption performance toward Hg(II), Cd(II) and Pb(II) by batch and fixed-bed column methods. J Environ Chem Eng 1(3):208–217
Stathi P, Dimos K, Karakassides MA et al (2010) Mechanism of heavy metal uptake by a hybrid MCM-41 material: surface complexation and EPR spectroscopic study. J Colloid Interface Sci 343(1):374–380
Talebi SM, Abedi M, Gheisari MM, Saber-Tehrani M (2010) Application of carbon dioxide supercritical fluid extraction of heavy metals from ambient aerosols. Asian J Chem 22(2):971–977
Xie D, Li C, Tang R et al (2014) Ion-exchange membrane bioelectrochemical reactor for removal of nitrate in the biological effluent from a coking wastewater treatment plant. Electrochem Commun 46:99–102
Yavuz Ö, Altunkaynak Y, Güzel F (2003) Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res 37(4):948–952
Yuan XZ, Meng YT, Zeng GM et al (2008) Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation. Colloids Surf, A 317(1–3):256–261
Zhang S, Bao A, Sun T et al (2015) PEI/Zr4+-coated nanopore for selective and sensitive detection of ATP in combination with single-walled carbon nanotubes. Biosens Bioelectron 63:287–293
Zhao HL, Fu LW, Zhao HY, Shi XW, Zhao SL, Liu HL (2016) Surface modification of amino-resin particles and its adsorption performance of Co2+ from simulated wastewater. Ion Exchange Adsorpt 32(2):097–107
Zheng Y, Wang A (2010) Removal of heavy metals using polyvinyl alcohol semi-IPN poly(acrylic acid)/tourmaline composite optimized with response surface methodology. Chem Eng J 162(1):186–193
Acknowledgements
This work is supported by the National Basic Research Program of China (2014CB748500), the National Natural Science Foundation of China (No. 91434110), the 111 Project of China (No. B08021) and the Fundamental Research Funds for the Central Universities of China. YW also acknowledges the support of Shanghai Pujiang Program (Grant No. 16PJD019).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shi, X., Fu, L., Wu, Y. et al. Functionalized dithiocarbamate chelating resin for the removal of Co2+ from simulated wastewater. Appl Water Sci 7, 4351–4360 (2017). https://doi.org/10.1007/s13201-017-0580-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-017-0580-y