Abstract
This research was conducted to investigate the treatment of hexavalent chromium (Cr(VI)) by iron powder (Fe(0)) columns of simulated permeable reactive barriers with and without calcium carbonate (CaCO3). Two columns filled with Fe(0) were used as Cr(VI) removal equipment running at a flow velocity of 10 ml/min at room temperature. After 200 days running of the two columns, the results showed that Fe(0) was an effective material for Cr(VI) reduction with an average removal rate of above 84.6%. The performance of Column 2 with CaCO3 was better than Column 1 without CaCO3 in terms of average Cr(VI) removal rate. The presence of CaCO3 buffered the increasing pH caused by Fe(0) corrosion in Column 2 and enhanced the removal rate of Column 2. Scanning Electron Microscopy (SEM) images of Fe(0) in the three stages of running of the two columns illustrated that the coat layer of Column 1 was a little thicker than that of Column 2. Energy-dispersive spectrometry (EDS) results showed that the surface of Fe(0) of Column 2 contained more chromium elements. Raman spectroscopy found that all iron oxide was generated on the Fe(0) surface of Column 1 and Column 2 and chromium class objects were only detected on Fe(0) surface in Column 2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cr is a common heavy metal pollutant from groundwater (Kjeldsen and Locht 2002). The main existence forms of Cr in groundwater are the oxidation states of Cr(III) and Cr(VI) (Barrera-Díaz et al. 2012). The nature of Cr is associated with its valence states of existence (Cohen et al. 1993). Cr(VI) is toxic and is easy to be absorbed and accumulated by organisms and makes them carcinogenic (Costa 1997; Costa and Klein 2006). In contrast, a trace of Cr(III) is beneficial for organisms and Cr(III) can form insoluble precipitates under slightly acidic or neutral conditions (Rai et al. 1989). Therefore, Cr(VI) is the main object to be removed due to its toxicity and carcinogenicity. It is considered to reduce Cr(VI) into Cr(III) and remove Cr(III) precipitates from the groundwater polluted by Cr(VI). Reagent-grade iron (Fe(0)) was successfully used to reduce Cr(VI) to Cr(III) under acidic conditions (Gould 1982; Gheju 2011). Batch and column experiments were performed to evaluate the potential of the two types of Fe(0) for the removal of Cr(VI) (Blowes and Ptacek 1992). Fe(0) has become the best material in repairing groundwater polluted by Cr(VI), because it has the characteristics of cheap and fast reaction rate (Fu et al. 2014). Since the late 1990s, a number of pilot-scale field permeable reactive barriers (PRBs) composed of granular Fe(0) and other materials were installed in situ in the reduction of Cr(VI) and trichloroethylene (TCE) (Blowes et al.1997; Puls et al. 1999; Puls 1999; Wilkin et al. 2005; Henderson and Demond 2007; Yang et al. 2007; Thiruvenkatachari et al. 2008). Although PRB bearing Fe(0) has been effectively used to treat Cr(VI), there is a major problem of PRB longevity. Some reports found a decrease in Fe(0) reactivity with reaction time (Eykholt et al. 1999; Mackenzie et al. 1999; Liang et al. 2005). Fe(0) reactivity has an important effect on PRB longevity and the decrease is attributed to the consumption of the reactive Fe(0) and the decline of reactive surface of Fe(0) due to the mineral precipitates (O’Hannesin and Gillham 1998; Vogan et al. 1999; Liang et al. 2000; Kamolpornwijit et al. 2003; Wilkin and Puls 2003; Li et al. 2006; Jeen et al. 2008). The minerals that precipitate on the Fe(0) surface depend on the groundwater geochemistry and can vary according to sites (Liang et al. 2000). Carbonate has been found to enhance (Johnson et al. 1996) or inhibit (Devlin and Allin 2005) reactivity depending on the groundwater conditions. It has also been found to enhance the reactivity initially and then inhibit it over long term (Klausen et al. 2003). CaCO3 is a conventional inorganic component in groundwater and its influence on the activity of Fe(0) is also very important. In this research, we focused on the influences of CaCO3 on Fe(0) corrosion. The objectives of this research were to investigate the effects of dissolved CaCO3 on Fe(0) reactivity toward Cr(VI) reduction, study Cr(VI) removal, solution pH change, Fe(0) powder surface materials through the columns experiments and illustrate the corrosion mechanism of Fe(0).
Materials and methods
Iron characterization
The main material in the experiment was iron powder with a size range of 325–425 µm and purity over 98.5%. Its loose loaded density was approximately 2.6 g/cm3. The impurities of iron included Mn, Si, C and S, the contents of which were 0.30, 0.11, 0.02 and 0.02%, respectively. In addition, in the iron there are a few materials insoluble in hydrochloric acid.
Groundwater sample
Potassium dichromate (K2Cr2O7) and CaCO3 of analytical grade were used as experimental reagents. The clean groundwater with added K2Cr2O7 or K2Cr2O7 and CaCO3 was used as polluted groundwater. The concentrations of Cr(VI) solution and CaCO3 solution were 10 mg/L and 50 mg/L, respectively.
Experimental system
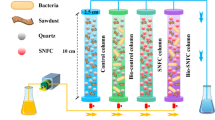
The photo of the two columns and the schematic of the experimental system are shown in Fig. 1. A laboratory-scale PRB system was developed using two similar continuous flow columns filled with Fe(0) powder to remove Cr(VI) of the polluted groundwater. The inner diameter of the columns was 10 cm, and the length of the columns was 80 cm. There were five sampling ports located at 10, 20, 30, 40 and 50 cm from the influent end of the columns. At the bottom of the columns, 5 cm-thick quartz sand was packed to support Fe(0) powder on the top of it. The corresponding solution was pumped into the columns from the bottom using a multi-channel peristaltic pump (BT-50EA). The influent flow velocity of the columns was designed as 10 ml/min. The influent of Column 1 contained 10 mg/L of Cr(VI), and that of Column 2 contained 10 mg/L of Cr(VI) and 50 mg/L of CaCO3. Samples were periodically collected from all sampling ports, including the influent and the effluent, and the Cr(VI) concentrations and pH of the samples were measured using an atomic absorption spectrophotometer (TAS-990 Super) and acidity meter (pHs-3E). The corrosion of the Fe(0) from columns was observed by scanning electron microscopy (SEM) (HITACHI), energy-dispersive spectrometry (EDS) and Raman spectroscopy (Invia 2000). Experiments were conducted at room temperature.
Results and discussion
Changes in pH
To study the pH changes in Column 1 and Column 2, the pH values of the water samples from ten sampling ports of two columns were monitored as the experimental system running. Figure 2 gives the results. Within 28 days of the running of the columns, the pH of the two columns rose fast, which illustrated that the reaction of Fe(0) and Cr(VI) was quick and there was little precipitation on the surface of the iron to reduce the activity of Fe(0). After 28 days, the pH of 10 cm from the influent end of the two columns did not change significantly. At this sampling spot, the pH of Column 2 was higher than that of Column 1, because the influent pH of Column 2 (pH 6.5) was higher than that of Column 1 (pH 6.9). After 28 days, the pH at 50 cm from the influent end of the two columns slowly rose and the pH of Column 1 was a little higher than that of Column 2. It was a consequence of the reaction of Fe(0) and Cr(VI). The equations of the reaction were as follows (Dries et al. 2005; Lai and Lo 2008):
In the equations, Fe(0) is oxidized to Fe(III), Cr(VI) is reduced to Cr(III) and the reactions form the hydroxyl ions; therefore, pH increases. After 28 days, some coating reduced the iron reactivity gradually formed on the iron, the reaction became slow and the consumption of hydrogen ions decreased, so the increasing trend of pH became slow. At the top of the column, there was more consumption of hydrogen ions, so the pH of the effluent was higher than that of the influent. pH of 50 cm from inffluent of Column 1 was a little higher than that of Column 2, because CaCO3 of Column 2 buffered the increasing pH.
Cr(VI) removal
During operation of the experimental system, the average concentrations and removal of Cr(VI) of effluent in columns were detected. The results are shown in Fig. 3. Before 28 days of running, the Cr(VI) concentration in the effluent was gradually increased both in Column 1 and Column 2. But the effluent Cr(VI) concentration of Column 2 was slightly lower than that of Column 1. After 28 days, the Cr(VI) concentration of Column 2 declined in general, and the Cr(VI) concentration of Column 1 fluctuated up and down. At 200 days, the Cr(VI) concentration of Column 1 was 1.31 mg/L and Cr(VI) removal rate was 86.9%; the Cr(VI) concentration of Column 2 was 0.89 mg/L and Cr(VI) removal rate was 91.1%. The results showed that Column 2 acquired better Cr(VI) removal than Column 1. This showed that a small amount of CaCO3 did not reduce the removal of Cr(VI), but improved efficiency of removal of Cr(VI). The addition of CaCO3 buffered the high pH caused by Cr(VI) reduction and iron corrosion, and thus the reduction reactions were enhanced. Specific reaction equations are shown as follows: CO3 2−+ H+→HCO3 −, HCO3 −+ Fe2+→FeHCO3 +, 2HCO3 −+Fe2+→Fe(HCO3)0. These materials will form compounds with the Fe2+ generated during iron corrosion, thus destabilizing the carbonate passive film and enhancing the corrosion process (Klausen et al. 2003).
SEM images of the iron surface
Figure 4 shows the SEM images of the iron powder. SEM images (magnification 500 and 3000) of the original iron are shown in Fig. 4a, b. According to the two pictures, there were many voids between the clean iron powder and no coating around the granular iron. Figure 4c, d give the SEM images of Column 1 after 100 day of column running. It was apparent from the pictures that a lot of flocculent and granular fines were attached to the surface of iron powder. This phenomenon was due to a number of precipitates produced during the reaction. Figure 4e, f were the pictures of iron powder of Column 2 after 200 day of column running. The pictures of the iron powder of Column 2 had a little different from Column 1. There were a large number of rod-shape and granular fines attached to the surface of granular iron. It showed that the two columns of iron powder generated different substances of different amounts.
EDS analysis of the iron surface
Surface elements of iron of different columns were analyzed by the energy-dispersive spectrometry (EDS). Figure 5 shows the EDS images of iron powder in Column 1 and Column 2. Figure 5a, b shows the scanning zones of iron powder and the image of the EDS of Column 1. Figure 5c, d shows the scanning zones of iron powder and the image of EDS of Column 2. From the images, it was clear that Column 1 and Column 2 contained the same elements of carbon, oxygen, silicon, calcium, chromium and iron. Among them, the sources of silicon were impurities of the iron or sand from the bottom of the columns, and carbon and calcium come from the influent. Oxygen and chromium come from potassium dichromate of the influent. The weight content and atom content of specific elements of different columns are shown in Table 1. Oxygen and chromium contents in Column 2 were higher than those of Column 1, which showed that potassium dichromate was used more by iron and the reaction rate was fast. The calcium content of Column 2 was higher than that of Column 1, because Column 2 had added CaCO3. The iron content of Column 1 was less than that of Column 2, which showed that there was a relatively quick reaction in Column 2.
Raman spectroscopy analysis of the iron surface
To know which substances were generated on the surface of iron, the iron surface was scanned by Raman spectroscopy. Figure 6a, b gives the pictures of the Raman spectroscopy of iron of Column 1 and Column 2. The Raman spectrum of the iron surface of Column 1 had peaks at 151, 241, 289, 399, 463, 537, 553, 657 and 705 cm−1, and the corresponding materials were CaCO3, α-Fe2O3, Fe3O4, γ-Fe2O3, α-FeOOH, δ-FeOOH. The Raman spectrum of the iron surface of Column 2 had peaks at 156, 310, 409, 458, 544, 600 and 662 cm−1, and the corresponding materials were CaCO3, α-Fe2O3, Fe3O4, γ-Fe2O3, β-FeOOH, Cr2O3 and CrO4 2−. Iron oxide consisted of a mixture of Fe2O3, Fe3O4 and FeOOH, and chromium oxide included Cr2O3 and CrO4 2−. The substances generated on the iron surface of the two columns had not much difference, which showed that the existence of CaCO3 did not change the types of products. We obtained similar conclusions with Gui et al. (2009). Precipitate formation is important to PRB technology because it governs the reactivity of the iron surface (Phillips et al. 2000).
Conclusions
After 200 days of running of equipment at a flow velocity of 10 ml/min at room temperature, the results testify that iron powder with a size range of 325–425 µm and purity over 98.5% was an effective material for Cr(VI) reduction regardless of the absence of CaCO3, and the average removal rate of Cr(VI) in the two columns was above 84.6%. But the presence of CaCO3 further increased Cr(VI) removal rate of Column 2 in the case of dissolved CaCO3 of 50 mg/l. The photos of SEM, EDS and Raman spectroscopy showed that through the reaction of Fe(0) and Cr(VI), iron oxide and chromium oxide were generated on the surface of iron. These materials on the iron surface play a key role in PRB longevity.
References
Barrera-Díaz CE, Lugo-Lugo V, Bilyeu B (2012) A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J Hazard Mater 223:1–12
Blowes DW, Ptacek CJ (1992) Geochemical remediation of groundwater by permeable reactive walls: removal of chromate by reaction with iron-bearing solids. In: Subsurface Restoration Conference, 3rd International Conference on Ground Water Quality Research, June, pp 21–24
Blowes DW, Ptacek CJ, Jambor JL (1997) In-situ remediation of Cr(VI)-contaminated groundwater using permeable reactive walls: laboratory studies. Environ Sci Technol 31(12):3348–3357
Cohen MD, Kargacin B, Klein CB, Costa M (1993) Mechanisms of chromium carcinogenicity and toxicity. Crit Rev Toxicol 23(3):255–281
Costa M (1997) Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit Rev Toxicol 27(5):431–442
Costa M, Klein CB (2006) Toxicity and carcinogenicity of chromium compounds in humans. Crit Rev Toxicol 36(2):155–163
Devlin JF, Allin K (2005) Major anion effects on the kinetics and reactivity of granular iron in glass encased magnet (GEM) batch reactor experiments. Environ Sci Technol 39(6):1868–1874
Dries J, Bastiaens L, Springael D, Agathos SN, Diels L (2005) Combined removal of chlorinated ethenes and heavy metals by zerovalent iron in batch and continuous flow column systems. Environ Sci Technol 39(21):8460–8465
Eykholt GR, Elder CR, Benson CH (1999) Effects of aquifer heterogeneity and reaction mechanism uncertainty on a reactive barrier. J Hazard Mater 68(1):73–96
Fu F, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205
Gheju M (2011) Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut 222(1–4):103–148
Gould JP (1982) The kinetics of hexavalent chromium reduction by metallic iron. Water Res 16(6):871–877
Gui L, Yang Y, Jeen S-W, Gillham RW, Blowes DW (2009) Reduction of chromate by granular iron in the presence of dissolved CaCO3. Appl Geochem 24(4):677–686
Henderson AD, Demond AH (2007) Long-term performance of zero-valent iron permeable reactive barriers: a critical review. Environ Eng Sci 24(4):401–423
Jeen SW, Blowes DW, Gillham RW (2008) Performance evaluation of granular iron for removing hexavalent chromium under different geochemical conditions. J Contam Hydrol 95(1):76–91
Johnson TL, Scherer MM, Tratnyek PG (1996) Kinetics of halogenated organic compound degradation by iron metal. Environ Sci Technol 30(8):2634–2640
Kamolpornwijit W, Liang L, West OR, Moline GR, Sullivan AB (2003) Preferential flow path development and its influence on long-term PRB performance: column study. J Contam Hydrol 66(3):161–178
Kjeldsen P, Locht T (2002) Removal of chromate in a permeable reactive barrier using zero-valent iron. IAHS Publication, pp 409–414
Klausen J, Vikesland PJ, Kohn T, Burris DR, Ball WP, Roberts AL (2003) Longevity of granular iron in groundwater treatment processes: solution composition effects on reduction of organohalides and nitroaromatic compounds. Environ Sci Technol 37(6):1208–1218
Lai KC, Lo IM (2008) Removal of chromium (VI) by acid-washed zero-valent iron under various groundwater geochemistry conditions. Environ Sci Technol 42(4):1238–1244
Li L, Benson CH, Lawson EM (2006) Modeling porosity reductions caused by mineral fouling in continuous-wall permeable reactive barriers. J Contam Hydrol 83(1):89–121
Liang L, Korte N, Gu B, Puls R, Reeter C (2000) Geochemical and microbial reactions affecting the long-term performance of in situ ‘iron barriers’. Adv Environ Res 4(4):273–286
Liang L, Moline GR, Kamolpornwijit W, West OR (2005) Influence of hydrogeochemical processes on zero-valent iron reactive barrier performance: a field investigation. J Contam Hydrol 78(4):291–312
Mackenzie PD, Horney DP, Sivavec TM (1999) Mineral precipitation and porosity losses in granular iron columns. J Hazard Mater 68(1):1–17
O’Hannesin SF, Gillham RW (1998) Long-term performance of an in situ ‘‘IronWall’’ for remediation of VOCs. Ground Water 36(1):164–170
Phillips DH, Gu BWDB, Watson DB, Roh Y, Liang L, Lee SY (2000) Performance evaluation of a zerovalent iron reactive barrier: mineralogical characteristics. Environ Sci Technol 34(19):4169–4176
Puls RW (1999) An in-situ permeable reactive barrier for the treatment of hexavalent chromium and trichloroethylene in ground water, vol 1. US Environmental Protection Agency, National Risk Management Research Laboratory
Puls RW, Paul CJ, Powell RM (1999) The application of in situ permeable reactive (zero-valent iron) barrier technology for the remediation of chromate-contaminated groundwater: a field test. Appl Geochem 14(8):989–1000
Rai D, Eary LE, Zachara JM (1989) Environmental chemistry of chromium. Sci Total Environ 86(1–2):15–23
Thiruvenkatachari R, Vigneswaran S, Naidu R (2008) Permeable reactive barrier for groundwater remediation. J Ind Eng Chem 14(2):145–156
Vogan JL, Focht RM, Clark DK, Graham SL (1999) Performance evaluation of a permeable reactive barrier for remediation of dissolved chlorinated solvents in groundwater. J Hazard Mater 68(1):97–108
Wilkin RT, Puls RW (2003) Capstone report on the application, monitoring, and performance of permeable reactive barriers for ground-water remediation: Volume 1: Performance evaluations at two sites (No. EPA/600/R-03/045A). NATIONAL RISK MANAGEMENT RESEARCH LAB ADA OK
Wilkin RT, Su C, Ford RG, Paul CJ (2005) Chromium-removal processes during groundwater remediation by a zerovalent iron permeable reactive barrier. Environ Sci Technol 39(12):4599–4605
Yang JE, Kim JS, Ok YS, Yoo KR (2007) Mechanistic evidence and efficiency of the Cr(VI) reduction in water by different sources of zerovalent irons. Water Sci Technol 5(1):197–202
Acknowledgements
We are extremely thankful to the National Natural Science Foundation of China for providing financial assistance to this research work (No. 51308274).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gao, Y., Liu, R. Removal of Cr(VI) from groundwater by Fe(0). Appl Water Sci 7, 3625–3631 (2017). https://doi.org/10.1007/s13201-016-0506-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0506-0