Abstract
In this study, the photocatalytic activity of polypyrrole-TiO2 nanocomposite was studied experimentally for the degradation of methylene blue (MB) dye under simulating solar light irradiation. To improve the photocatalytic activity of TiO2 under sunlight irradiation, conducting polymers such as polypyrrole (PPy) and its derivatives are generally used as photosensitizers. The PPy-TiO2 nanocomposite was prepared by the chemical oxidative polymerization method. The prepared nanocomposite showed better photocatalytic activity than bare TiO2 under sunlight irradiation for the degradation of MB dye. The prepared nanocomposite was subjected to characterization techniques such as SEM-EDAX, FT-IR, UV-DRS, XRD, TGA and PL spectral analysis. Different influencing operating parameters like initial concentration of dye, irradiation time, pH and amount of PPy-TiO2 nanocomposite used have also been studied. The optical density of the dye degradation was measured by UV–Visible spectrophotometer. The repeatability of photocatalytic activity was also tested. A plausible mechanism was proposed and discussed on the basis of experimental results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In current years, pollution from dye wastewater has become a severe ecological problem due to the enormous and increasing uses of a variety of dyes (Kangwansupamonkon et al. 2010), When these compounds are discharged to the major water bodies without any previous treatment, they can cause disaster to the ecological equilibrium in the environment as these molecules have carcinogenic and mutagenic properties in the direction of water organisms and thus produce treat to a human being at the end of these food chains (Muthirulan et al. 2012a, b). Methylene blue is a heterocyclic aromatic substance having IUPAC name 3,7-bis(dimethylamine)–phenothiazine-5-iumchloride with the molecular formula C16H18N3SCl. It has many uses in a selection of different fields, such as environmental science and chemistry (Eskizeybek et al. 2012). The physico-chemical properties of methylene blue (MB) dye are illustrated in Table 1.
Methylene blue dye is a cationic dye. The pzc of TiO2 is 6.8. For pH values less than pHpzc, the surface becomes positively charged, and for pH values greater than pHpzc the TiO2 surface will be negatively charged. Hence, it is absorbed the positively charged MB dye species easily in basic pH.
Semiconductor photocatalysis shows a potential approach for solving worldwide environmental pollution issues. Titanium dioxide (TiO2), as an important semiconductor, has been widely investigated in the photocatalytic field, due to its abnormal chemical and physical behaviours. On the other hand, the high charge recombination rate in TiO2 considerably limits its photocatalytic application (Ibrahim and Halim 2008; Maeda 2012; Ochiai and Fujishima 2012; Park et al. 2013).
To develop the photocatalytic activity of TiO2, there has been significant progress in the construction of new efficient materials by coupling TiO2 with other organic, inorganic, polymeric materials, such as TiO2/zeolite, TiO2/activated carbon, TiO2/Al2O3, TiO2/SiO2 and TiO2/polymer materials. These composites really show photocatalytic development to a certain level. Among them, TiO2/polymer composite paying more attention than others. The reason is that apart from outstanding mechanical property and large surface area, polypyrrole has electrical and electronic properties.
In recent times, some studies have been published on the combination of conductive polymers and TiO2 to get better performance of UV light and sunlight activities (Muthirulan et al. 2013; Hou et al. 2011; Xu et al. 2012; Zhang et al. 2008; Denga et al. 2012; Zhang et al. 2006; Li et al. 2008; Wang et al. 2008; Kandiel et al. 2009; Wang et al. 2009). Moreover, many conjugated polymers also are efficient electron donors and good hole transporters upon visible light excitation. Therefore, conjugated polymers with large band gap inorganic semiconductors obtain awareness for optical, electronic, photocatalytic and photoelectric conversion applications.
In spite of many reward of TiO2, its photocatalytic water-splitting efficiency under solar energy is still somewhat low, mainly owing to the following reasons; First, the photo-generated electrons in the CB of TiO2 can recombine with the VB holes rapidly to liberate energy in the outward appearance of blocked heat or photons; Second, the decomposition of water into hydrogen and oxygen is a chemical reaction with large positive Gibb’s free energy (ΔG = 237kJ/mol), thus the backward reaction (recombination of hydrogen and oxygen into water) easily proceeds; Third, the band gap of TiO2 is about 3.2 eV. Since UV light only accounts for in the region of 4 % of solar energy, while visible light contributes about 50 %, the lack of ability to make use of visible light confines the effectiveness of TiO2 in solar photocatalytic hydrogen production.
To solve the above-mentioned trouble and to make solar photocatalytic by TiO2 sufficient, constant efforts have been made by adding electron donating organic polymer (PPy). Adding electron donors or sacrificial reagents to react with the photo-generated VB holes is an effective quantify to enhance the electron–hole separation, resulting in higher quantum efficiency. However, the disadvantage of this system is required to continuously add electron donors (organic molecules) to maintain the reaction since they will be inspired during the photocatalytic reaction. (Li et al. 2010; Ufana and Ashraf 2011; Arenas et al. 2013).
In this manuscript, effective PPy-TiO2 composites have been fabricated by chemical oxidative polymerization method. The prepared PPy-TiO2 nanocomposite was characterized by SEM, XRD, UV-DRS, FT-IR and PL studies. The photodegradation studies were carried out by the prepared PPy-TiO2 nanocomposite. The prepared PPy-TiO2 nanocomposite, a novel solar light driven catalyst PPy-TiO2, shows high photocatalytic activity as compared to TiO2.
Experimental
Chemicals and reagents
All these reagents are of AR grade and used without further purification. The list of chemicals is illustrated in Table 2.
Preparation of conductive PPy-TiO2 nanocomposites
The preparation of conductive PPy-TiO2 nanocomposite is illustrated in Scheme 1. Chemical oxidative polymerization of PPy was performed in the presence of negatively charged TiO2 nanoparticles (different wt%, i.e. 0.5, 1.0, 1.5 and 2.0 wt%) using ammonium persulfate as an oxidant. A typical procedure is outlined: Pyrrole (5 mmol) was added to 500 ml of 0.1 M Sulphuric acid solution. Then, the TiO2 nanoparticles (different wt%, i.e. 0.5, 1.0, 1.5 and 2.0) were added with continuous stirring. Then, it was kept under 5 °C cooled condition. Then, 20 ml of Ammonium persulfate solution was added drop by drop. The green coloured solution was obtained. After constant stirring, the precipitation occurred in dark colour. The obtained product was washed with distilled water to remove the remaining ammonia solution. Finally, the product was dried out at room temperature at overnight.
Instrumental analysis
UV–visible spectrophotometer (DRS) was recorded using “SHIMADZU” model: UV 2450, FT-IR spectrum was recorded using “SHIMADZU” (Model: 8400S). The crystallographic structures of the materials were determined by high-resolution powder diffractometer model—RICH SIEFRT & CO with Cu as the X-ray source (λ = 1.5406 × 10−10 m). The surface morphology of the sample was recorded using scanning electron microscopy (SEM-EDAX) (Model: FEG Quantum 250). The photoluminescence (LS 45) was recorded using a Perkin Elmer spectra. The thermal stability of the material was recorded using Thermogravimetric Analyser (Model : TG/DTA 6200) “SII nanotechnology”.
Photodegradation studies
The photocatalytic activity of efficient PPy-TiO2 nanocomposites was estimated by measuring the decolorization rates of MB. In our experiments, 50 ml of MB and 20 mg of catalysts were kept under dark room to get adsorption desorption equilibrium. Then, it suspended into sunlight irradiation. At given intervals of enlightenment, a sample of the catalyst particulate was collected and centrifuged. The supernatant was analysed by spectrophotometer (ELICO SL 207 MINI SPEC) at λ max = 665 nm. The determined absorption was changed to concentration through the standard curve method. From that experiment, we work out the percentage removal of dye.
The amount of removal of the MB dye, in terms of percentage removal, has been calculated using the following relationship:
where C i and C f are the initial and final concentrations of dye (ppm) at a given time.
The reaction was carried out the time between 12.00 and 1.30 p.m. The intensity of Sun light was measured by Lux Meter (TES 1332A digital Lux meter). In this work, we did not consider the water evaporation and consequent solution concentration. The processes will be included in our future studies.
Results and discussion
Surface morphology of PPy-TiO2 nanocomposite (SEM-EDAX)
The morphology of the composite strongly depends upon the character as well as the method of preparation. The surface morphology of the prepared PPy-TiO2 nanocomposite was examined by SEM-EDAX. Figure 1a shows the surface morphology Polypyrrole having flower like structure. Figure 1b represents that the surface morphology of TiO2 is a ball-like round shape deposited on the surface of polypyrrole. Based on these conclusions in PPy-TiO2 nanocomposite, the TiO2 particles deposited on the outside of polypyrrole (Hongyu et al. 2010; Guoquan et al. 2011).
FT-IR studies
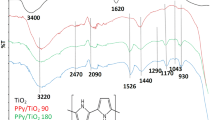
The molecular structure and functional group of PPy and PPy-TiO nanocomposites (0.5, 1.0, 1.5, 2.0 wt%) were determined by FTIR spectrum. Figure 2 gives the typical FTIR spectra of PPy polymer, and the bands at 1552.15 and 1635.13 cm−1 are attributed to the stretching vibrations of C=C and C=N in the phenazine ring, respectively. The peaks at 1400.22 and 1100 cm−1 are attributed to the C–N stretching in the benzenoid and quinoid imine units. In addition, the bands at 520.78 cm−1 which are the description of C–H out-of plane bending vibrations of benzene nuclei of PPy. The FTIR spectra of the PPy-TiO2 nanocomposites with different wt% TiO2 represent the same characteristic peaks of PPy polymer. The peak in the region of 1100 cm−1 is related with vibrational modes of N=Q=N (Q refers to the pyrrole ring), representing that PPy is formed in the composite. But the stretching is decreased with the increase in the loading amount of TiO2. The results of FTIR spectra of PPy agree well with the earlier reports (Xiaofang et al. 2006; Deivanayaki et al. 2012; Mi et al. 2009; Chougulea et al. 2012, 2013; Chatterjee et al. 2013; Etelino et al. 2013; Juraj et al. 2013).
UV–vis DRS studies of PPy-TiO2 nanocomposite
The optical properties of PPy-TiO2 nanocomposites investigated by UV-DRS spectroscopy are shown in Fig. 3a. The band gap energies of PPy-TiO2 can be estimated from the wavelength values equivalent to the junction point of the perpendicular and parallel parts of the spectra.
From that spectra, the polymer expands the absorption range of the TiO2 from 400 nm to over 550 nm. In fact, the spectra of PPy-TiO2 were the spectra of polymer PPy adsorbed onto the surface of the powders. It is an essential condition for photo-sensitization that the photosensitizer be adsorbed onto the surface of semiconductor. PPy-TiO2 seemed to be capable of responding to visible light.
The position of the fundamental absorption edge of PPy-TiO2 is determined using the equation:
where α, h, υ, E g and A are the absorption coefficients, plank constant, light frequency, band gap and a constant, respectively. The n value depends on the transition characteristics. The E g value can be approximated by extrapolating the straight portion of the (αhυ)2 − (hυ) plot shown in Fig. 3b. The obtained E g value occurs between 2.22 and 2.23 e V for PPy-TiO2, respectively. The band gap value of 2.0 wt% TiO2-PPy nanocomposite was smaller when compared to other components as shown in Table 3. The band gap values estimated are somewhat smaller than the reported values which can be accredited to wide particle size distribution and surface defects (Rohit et al. 2013).
X-ray diffraction pattern of PPy-TiO2 nanocomposite
The crystallographic construction of obtained PPy-TiO2 nanocomposite was evidenced by XRD measurements. Figure 4a shows the XRD pattern of TiO2 and Fig. 4b shows the XRD pattern of PPy/TiO2 nanocomposites. The XRD peaks denote the arrangement of PPy-TiO2 nanocomposite. The respected peaks occur at 25.3°, 26.2°, 36.0°, 38.2°, 48.9°, 54.3°, 56.6°, 63.1°, 69.9°. In addition, it can be prominent due to the TiO2 deposited on the surface of polypyrrole (Hasani and Hossein 2013; Yucheng et al. 2013).
The mean crystalline sizes of PPy-TiO2 nanocomposites are designed using Scherrer’s formula:
where λ is the wavelength of the X-ray radiation (λ = 1.54 × 10−9nm), and k is the Scherrer constant (k = 0.89)
Θ is the diffraction angle and β is the line width at half-maximum height of the most intense peak. Based on the XRD results, the crystalline range of PPy-TiO2 nanocomposites was in the order of 10-20 nm. The results are in good agreement with the SEM images.
Photoluminescence spectra
To study the cause for the higher photocatalytic activity of PPy-TiO2 nanocomposites, PL spectra of samples have been taken, as shown in Fig. 5. The rate of recombination of e−/h+ pairs may also be a key factor disturbing the photoactivity of TiO2. Probably, the TiO2 photocatalysts show a broad PL emission band. The peak intensities of the PL spectra of PPy-TiO2, demonstrating that the electron—hole recombination rate of self—trapped excitation in TiO2, are reduced by the introduction of PPy. The significant PL quenching of TiO2 can be observed after combination with PPy, representing the efficient transfer of photo-generated electron from the TiO2 to PPy, most important to the enhanced photocatalytic activity of the PPy/TiO2 nanocomposites (Wang et al. 2010).
Thermogravimetric analysis
Figure 6 displays the thermogravimetric analysis of Pure PPy, 1.0 wt% TiO2-PPy and 2.0 wt% TiO2-PPy nanocomposites under N2 atmosphere with the heating rate 20 °C/min. As observed from Fig. 6, it demonstrates that the thermal stability of 2.0 wt% TiO2-PPy is much advanced than that of other composites in the temperature range of 30–800 °C. The first weight loss occurred at 100 °C due to the evaporation of remaining water molecule. The second weight loss occurs between 110 and 250 °C. This demonstrates the structural decay of PPy. Finally, the third weight loss occurs at 250–730 °C. This is due to the skeletal degradation of PPy. In contrast, 2.0 wt% TiO2-PPy nanocomposites have greater thermal stability than other two nanocomposites. The thermogram shows that the TiO2 nanoparticle lost about 11.9 % weight owing to the loss of polymer coatings on the surface of the nanoparticle.
Effect of concentration of dye
The outcome of initial concentration of Methylene Blue dye on the photocatalytic activity of photocatalysts (TiO2 and PPy-TiO2 nanocomposites) was carried out under solar light irradiation within the concentration range of 10–60 ppm and constant amount of catalyst and irradiation time and the results are shown in Fig. 7. These results indicated that PPy-TiO2 nanocomposites photocatalyst showed a high efficiency for the photocatalytic degradation of MB in the presence of solar light irradiation than TiO2.
When increasing the dye concentration from 10 to 20 ppm, the percentage decoloration of MB dye was decreased at 90–5 %. Finally, the degradation quickly decreases for 60 ppm (25 %). This may be due to:
-
1.
higher dye concentration might serve as an inner filter shunting the photons away from the catalyst surface;
-
2.
non-availability of oxidative free radicals;
-
3.
inverse effect is explained that if the dye concentration increases in the treating solution, large amount of dye molecules is adsorbed on catalyst surface active site. The surface active sites to contribute in the degradation reaction will help to enlarge the degradation efficiency. Due to the increase in dye concentration, there is no space for the formation of OH· radicals. So at last there is a drop in degradation efficiency. Hydroxyl radical is the strongest oxidizing agent promoting the degradation rate. According to the Beer–Lambert law, as the initial dye concentration increases, the pathway distance end to end of photon incoming the solution decreases consequently resulting in lower photo degradation rate (Zhao et al. 2012; Wang et al. 2012; Selvam et al. 2007).
Effect of dose variation
The influence of dose on the degradation of Methylene Blue has been investigated by PPy-TiO2 nanocomposite. This percentage removal of dye using PPy-TiO2 nanocomposites was higher than TiO2 (Fig. 8). The increase in the efficiency seems to be due to the increase in the whole surface area, namely the number of active sites obtainable for the photo catalytic reaction as the quantity of photo catalyst increased. When photocatalyst is added in excess, the degradation of dye molecules remains constant. The reason for this is the deceased light penetration, the increased light scattering and the slaughter in surface area occasioned by agglomeration at high solid concentration (Baruah et al. 2010).
Effect of time variation
The degradation of methylene blue was exponential with time. The degradation rate constant k for methylene blue was obtained from the plot in Fig. 9 using
where C o and C f are the initial and final concentrations of dye and t is the irradiation of time.
The degradation rate constants are similar for both the sample with decayed methylene blue fraction of 93 % leading to enlightenment for 90 min (Kansal et al. 2007).
UV–vis absorption spectrum of MB with different reaction time under solar light irradiation in the presence of PPy-TiO2 nanocomposites is illustrated in Fig. 10. Solar light irradiation leads to decreases in absorbance in MB in the presence of PPy-TiO2 nanocomposites and the decrease of the absorption intensities indicated that the dye has been degraded. As can be seen in figure, the departure of the distinguishing band of MB dye at 665 nm after 90 min under solar light irradiation indicates that Methylene Blue has been degraded completely.
The value of ln(C 0/C t) is plotted against time (in min) and the plots are found to be linear. From the slope, the rate constants were considered for the degradation of MB dye in the presence and absence of PPy. The pseudo first-order rate constant (k min−1) values of TiO2 and PPy-TiO2 system can be found out from the Fig. 9. The degradation efficiency of the MB dye PPy-TiO2 nanocomposite is higher than that of TiO2 as shown in Fig. 11.
Effect of pH variation
To study the effect of pH on the decolorization efficiency, experiments were carried out at various pH values, ranging from 3 to 11 for constant dye concentration (10 ppm) and catalysts loading (Fig. 12). It has been experimental that the decolorization efficiency increases with increasing pH exhibiting a maximum rate of degradation at pH 11 and 9. The zero point charge for TiO2 is 6.25. The pH increases from 7 to 11. The plane of the catalyst will become negatively charged. So the cationic dye (MB) easily attached to the catalyst surface. Finally, the percentage removal of dye increased. In contrast, at acidic condition the degradation efficiency will be decreased (Madhusudhana et al. 2012).
Mechanism
Photocatalytic mechanism of PPy-TiO2 nanocomposites can be described as below. The photocurrent values obtained from PPy to TiO2 nanocomoposite are higher than TiO2. PPy is a p-type narrow band gap semiconductor when compared with other inorganic semiconductors (TiO2, SrTiO3, ZnO) having n-type narrow band gap semiconductor. The lowest vacant molecular orbital level of PPy is positioned in an actively higher location than the conduction band edge of TiO2. Consequently, when the system was illuminated, the excited electrons of PPy in the highest engaged molecular orbital level could move to the LUMO level, then these electrons could be transferred into the conduction band of the TiO2. Thus, more and extra photo energized electrons formed in TiO2 and the combination of the semiconductors (p–n junction) should have a beneficial role in getting better charge separation. As a result, this scheme delivers important photo responses in this intersection.
The above-discussed mechanism is illustrated in Scheme 2.
Reuse of catalysts
The reproducibility of the photocatalytic degradation activity of a 0.02 g sample PPy-TiO2 nanocomposite performed on a 10 ppm MB dye solution (50 ml) is expressed as the number of cycles, where the photocatalytic degradation of PPy-TiO2 nanocomposite was reduced from 93 % on the first usage to 82, 29 and 20 % after the second, third and fourth cycles of reuse, respectively. This reduced dye photodegradation activity is in accord with the appearance of recycled PPy-TiO2 nanocomposite, which was clearly changed in the morphology of the PPy-TiO2 nanocomposites due to the photodegradation.
Conclusions
PPy-TiO2 nanocomposite was effectively prepared by the chemical oxidative polymerization method. The results from SEM analysis consistently specify that the TiO2 has been deposited on Polypyrrole atmosphere. The FT-IR results show the major functional group present in PPy-TiO2 nanocomposites. The UV–vis diffuse reflectance spectra confirm that the tailored catalyst immersed more photons under visible light irradiation. The TGA spectra indicate the thermal stability of PPy-TiO2 nanocomposites. The MB dye was effectively degraded by PPy-TiO2 nanocomposite under solar light irradiation. The fraction removal of dye degradation was higher (93 %) in PPy-TiO2 nanocomposite than TiO2.
References
Arenas MC, Fernando Rodríguez-Núnez L, Domingo R, Omar Martínez A, Martínez-Alonso C, Castaño VM (2013) Simple one-step ultrasonic synthesis of anatase titania/polypyrrole nanocomposites. Ultrason Sonochem 20:777–784
Baruah S, Jaisai M, Imani R, Nazhad M, Dutta J (2010) Photocatalytic paper using zinc oxide nanorods. Sci Technol Adv Mater 11:1–7
Chatterjee S, Shit A, Nandi AK (2013) Nanochannel morphology of polypyrrole-ZnO nanocomposites towards dye sensitized solar cell application. J Mater Chem A (1):12302–12309
Chougulea MA, Dalavib DS, Mali S, Patilb PS, Moholkarb AV, Agawanec GL, Kimc JH, Shashwati S, Patila VB (2012) Novel method for fabrication of room temperature polypyrrole—ZnO nanocomposite NO2 sensor. Measurement S0263-22410, 0193-5
Chougule MA, Khuspe GD, Shashwati S, Patil VB (2013) Polypyrrole–ZnO nanohybrids: effect of CSA doping on structure, morphology and optoelectronic properties. Appl Nanosci (3):423–429
Deivanayaki S, Ponnuswamy V, Jayamurugan P, Ashokan S (2012) The structure and properties of polypyrrole/titaniumdioxide nanospheres of various dopant percentages by Chemical oxidation method. Elixir Polymer 49B:10182–10185
Denga F, Li Y, Luob X, Lixia Y, Tu X (2012) Preparation of conductive polypyrrole/TiO2 nanocomposite via surface molecular imprinting technique and its photocatalytic activity under simulated solar light irradiation. Colloid Surf A: Physicochem Eng Aspects 395:183–189
Eskizeybek V, Fahriyesari B, Gulce H, Gulce A, Ahmet A (2012) Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of MB and MG dyes under UV and natural sunlights irradiations. Appl Catal B: Environ 119–120:197–206
Etelino F, Melo D, Kleber G, Alves B, Severinon A, Celso J, de Melo P (2013) Synthesis of fluorescent PVA/polypyrrole-ZnO nanofibers. J Mater Sci 48:3652–3658
Guoquan Z, Shuai W, Sha Z, Lei F, Guohua C, Fenglin Y (2011) Oxidative degradation of azo dye by hydrogenperoxide electrogenerated in situ on anthraquinonemonosulphonate/polypyrrole composite cathode with heterogeneous CuO/γ-Al2O3 catalyst. Appl Catal B: Environ 106:370–378
Hasani T, Hossein E (2013) Removal of Cd (II) by using polypyrrole and its nanocomposites. Synth Met 175:15–20
Hongyu M, Xiaogang Z, Youlong X, Fang X (2010) Synthesis, characterization and electrochemical behavior of polypyrrole/carbon nanotube composites using organometallic-functionalized carbon nanotubes. Appl Surf Sci 256:2284–2288
Hou J, Cao R, Jiao S, Zhu H, Kumar RV (2011) PANI/Bi12TiO20 complex architectures: controllable synthesis and enhanced visible-light photocatalytic activities. Appl Catal B: Environ 104:399–406
Ibrahim UG, Halim AA (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol C: Photochem Rev 9:1–12
Juraj D, Martin K, Gabriela B, Martin K, Iva M (2013) Electrochemical fabrication and characterization of porous silicon/polypyrrole composites and chemical sensing of organic vapors. Int J Electrochem Sci 8:1559–1572
Kandiel TA, Dillert R, Bahnemann DW (2009) Enhanced photocatalytic production of molecular hydrogen on TiO2 modified with Pt: polypyrrole nanocomposites. J Photochem Photobiol Sci 8:683–690
Kangwansupamonkon W, Jitbumpot W, Sudakiatkamjornwong C (2010) Photocatalytic efficiency of TiO2/poly[acrylamide-co-(acrylic acid)] composite for textile dye degradation. Polym Degrad Stab 95:1894–1902
Kansal SK, Singh M, Sud D (2007) Studies on photodegradation of two commercial dyesin aqueous phase using different photocatalyst. J Hazard Mater 141:581–590
Li XY, Wang DS, Cheng GX, Luo QZ, An J, Wang YH (2008) Preparation of polyaniline-modified TiO2 nanoparticles and their photocatalytic activity under visible light. Appl Catal B: Environ 81:267–273
Li Q, Zhang C, Li J (2010) Photocatalysis and wave-absorbing properties of polyaniline/TiO2 microbelts composite by in situ polymerization method. Appl Surf Sci 257:944–948
Madhusudhana N, Yogendra K, Mahadevan M (2012) A comparative study on photocatalytic degradation of violet GL2B azo dye using CaO and TiO2 nanoparticles. Int J Eng Res Appl 2(5):1300–1307
Maeda K (2012) Photocatalytic water splitting using semiconductor particles: history and recent developments- Invited review. J Photochem Photobiol C: Photochem Rev 12:237–268
Mi O, Ru B, Yi X, Cheng Z, Chun MA, Man W, Hong-zheng C (2009) Fabrication of polypyrrole/TiO2 nanocomposite via electrochemical process and its photoconductivity. Trans Nonferrous Met Soc China 19:1572–1577
Muthirulan P, Meenakshi Sundaram M, Kannan N (2012a) Beneficial role of ZnO photocatalyst supported with porous activated carbon for the mineralization of alizarin cyanin green dye in aqueous solution. J Adv Res. doi:10.1016/j.jare.2012.08.005
Muthirulan P, Nirmala Devi C, Meenakshi Sundaram M (2012b) Synchronous role of coupled adsorption and photocatalytic degradation on CAC-TiO2 composite generating excellent mineralization of alizarin cyanine green dye in aqueous solution. Arab J Chem 1:1–7
Muthirulan P, Kannan Nirmala Devi C, Meenakshi Sundaram M (2013) Facile synthesis of novel hierarchical TiO2@Poly(o-phenylenediamine) core–shell structures with enhanced photocatalytic performance under solar light. J Environ Chem Eng 1:620–627
Ochiai T, Fujishima A (2012) Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification-invited review. J Photochem Photobiol C: Photochem Rev 13:247–262
Park H, Park Y, Kim W, Choi W (2013) Surface modification of TiO2 photocatalyst for environmental applications: invited review. J Photochem Photobiol C: Photochem Rev 15:1–20
Rohit B, Madhulika S, Bahadur D (2013) Visible light-driven nanocomposites (BiVO4/CuCr2O4) for efficient degradation of organic dye+. Dalton Trans 42(19):6736–6744
Selvam K, Muruganandham M, Muthuvel I, Swaminathan M (2007) The influence of inorganic oxidants and metal ions on semiconductor sensitized photodegradation of 4-fluorophenol. J Chem Eng 128:51–57
Ufana R, Ashraf SM (2011) Semi-conducting poly(1-naphthylamine) nanotubes: a pH independent adsorbent of sulphonate dyes. Chem Eng J 174:546–555
Wang DS, Wang YH, Li XY, Luo QZ, An J, Yue JX (2008) Sunlight photocatalyticss activity of polypyrrole: TiO2 nanocomposites prepared by ‘insitu’ method. Catal Commun 9(1162–1166):7
Wang D, Zhang J, Luo Q, Li X, Duan Y, An J (2009) Characterization, photocatalytic activity of poly(3-hexylthiophene)-modified TiO2 for degradation of methyl orange under visible light. J Hazard Mater 169:546–550
Wang Y, Shi R, Lin J, Zhu Y (2010) Significant photocatalytic enhancement in methylene blue degradation of TiO2 photocatalysts via grapheme-like carbon in situ hybridisation. Appl Catal B: Environ 100:179–183
Wang WS, Wang DH, Qu WG, Lu LQ, Xu AW (2012) Large ultrathin anatase TiO2 nanosheets with exposed 001 facets on grapheme for enhanced visible light photocatalytic activity. J Phys Chem C 116:19893–19901
Xiaofang L, Qidong Z, Xin LC, Dajun W, Wanjin Z, Wang C, Wei Y (2006) Prepartion and characterization of polypyrrole/TiO2 coaxial nanocables. Macromol Rapid Commun 27:430–434
Xu S, Gu L, Yang KH, Song Y, Jiang L, Dan Y (2012) The influence of the oxidation degree of poly(3-hexylthiophene) on the photocatalytic activity of poly(3-hexylthiophene)/TiO2 composites. Sol Energy Mater Sol Cells 96:286–291
Yucheng Y, Junwei W, Jianhong W, Xiong R, Shi J, Chun-Xu P (2013) Polypyrrole-decorated Ag-TiO2 nanofibers exhibiting enhanced photocatalytic activity under visible light illumination. ACS Appl Mater Interfaces 5(13):6201–6207
Zhang L, Liu P, Su Z (2006) Preparation of PANI/TiO2 nanocomposites and their solidphase photocatalytic degradation. Polym Degrad Stab 91:2213–2219
Zhao D, Sheng G, Chen C, Wang X (2012) Enhanced photocatalytic degradation in methylene blue under visible irradiation on grapheme@TiO2 dyade structure. Appl Catal B: Environ 111:303–308
Acknowledgments
The authors thank the University Grants Commission, New Delhi for the Financial support in the form of Major Research Project (MRP) Grant. The authors also thank Ayya Nadar Janaki Ammal College, Sivakasi for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sangareswari, M., Meenakshi Sundaram, M. Development of efficiency improved polymer-modified TiO2 for the photocatalytic degradation of an organic dye from wastewater environment. Appl Water Sci 7, 1781–1790 (2017). https://doi.org/10.1007/s13201-015-0351-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0351-6