Abstract
Mutualisms are of fundamental ecological importance, but risk breaking down if one partner stops paying the costs yet still takes the benefits of the interaction. To prevent such cheating, many mutualisms have mechanisms that lower the fitness of uncooperative symbionts, often termed host sanctions. In mutualisms where the interacting partners are species-specific, we would expect to see coevolution of the levels of host sanctions and partner cooperation across species-pairs. In the mutualism between fig trees and their species-specific pollinating fig wasps, host sanctions vary greatly in strength, and wasp cooperation levels vary accordingly. Here I show experimentally that in Panamanian Ficus perforata (section Urostigma, Americana) there are fitness costs for wasps that do not pollinate. These fitness costs are caused by a combination of abortions of unpollinated figs and reduced proportion of wasp larvae that successfully develop to adults. The relative fitness of wasps that do not pollinate compared to wasps that pollinate is 0.59, leading to the intermediate sanction strength 0.41. Next, by screening pollinators of F. perforata I found that 1.9% of wasp individuals in natural populations failed to carry pollen. Across five actively pollinated Neotropical fig species and their pollinators, fig species with stronger host sanctions had fewer uncooperative wasps, as would be expected if sanctions promote cooperation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mutualisms, interactions between two species where both benefit from the interaction, are of fundamental ecological importance. For example, most forest trees are assisted by mycorrhizal fungi to absorb nutrients from soil, most animals are assisted by gut microbes to absorb nutrients from food, and over 90% of flowering plants rely on pollinators to set seed (Parniske 2008; Ollerton et al. 2011; Foster et al. 2017). Because the two partners in a mutualism often trade costly services, there is the risk that one of the partners will stop providing the service, yet still take the benefits. Such cheaters would gain a relative fitness advantage and would risk breaking down the mutualism (Bull and Rice 1991; Sachs et al. 2004; West et al. 2007). Therefore, mutualisms require mechanisms that prevent such cheating and promote cooperation (Ågren et al. 2019, but see Frederickson 2017 for an alternative view).
Empirical researchers in many mutualistic systems have found that symbionts (here used to mean the smaller of the two partners) that are forced to not cooperate with their host (here used to mean the larger of the two partners) often have lower fitness than those that do cooperate. These mechanisms are often referred to as host sanctions; while the underlying mechanism is likely one of preferential resource allocation to more productive plant parts, the effect on the symbiont is that symbionts that are more cooperative achieve higher fitness (Kiers et al. 2003; Kiers et al. 2011; Jandér and Herre 2016). For example, rhizobia that do not provide their host legume with nitrogen get less benefits in return, and mycorrhizal fungi that do not provide their host plant with phosphorous get less carbohydrates in return (Kiers et al. 2003; Kiers et al. 2011). While these two mutualisms are ubiquitous, other mutualisms can be ideal model systems for studying why partners in a mutualism do not cheat. One such model system is the mutualism between fig trees and their pollinating fig wasps.
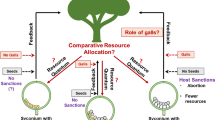
The fig mutualism is a keystone resource for tropical forests and their frugivorous animals. Fig trees fruit year round, and are therefore an important food source for frugivorous animals during gaps in seasonal fruiting of other plant species (Shanahan et al. 2001). To fruit, wild fig trees need to be pollinated by fig wasps. Fig trees and fig wasps are dependent on each other for reproductive success: while trees can only be pollinated by fig wasps, fig wasps can only lay their eggs in fig flowers (Herre et al. 2008; Fig. 1). Compared to the more generalist systems of legumes and rhizobia, and plants and mycorrhizae, one of the outstanding advantages of studying the fig mutualism is that each fig species is pollinated by one or a few species-specific pollinator fig wasps ((Berg 1989; Cruaud et al. 2012; Satler et al. 2019); although a few rare cases with shared pollinators exist (Machado et al. 2005; Wei et al. 2014)). This species-specificity makes the system ideal for studying coevolutionary questions. Additionally, because there are over 750 species of fig trees globally, the system allows comparative studies (Cruaud et al. 2012; Rasplus et al. 2020; Wang et al. 2021). Two thirds of fig wasp species pollinate actively, ie they actively use their front legs to first collect pollen from their natal fig and place it into specialized pollen pockets, then actively use their front legs to disperse this pollen onto the flowers in the fig where they oviposit (Frank 1984; Cruaud et al. 2012). Active pollination is beneficial for the tree but costly for the wasp; it requires specialized structures and behaviour, and takes time that otherwise could be used to lay eggs (Kjellberg et al. 2001; Jandér and Herre 2010; Jandér and Steidinger 2017; Pellmyr et al. 2020). Hence, we expect there to be mechanisms that encourage wasps to continue this costly mutualistic behaviour.
(a) A female pollinator in the process of entering a receptive (flowering) fig of F. perforata. (b) Each receptive fig of F. perforata is smaller than half a pea and contains on average 168 flowers; each flower can develop into either a wasp gall or a seed. The red part is the entrance to the fig. (c) After about 25 days the wasp offspring emerge from their galls, collect pollen, then leave their natal fig. Soon after wasp departure the figs swell to the size of blueberries, sweeten, and turn deep red, to mainly be dispersed by birds. Photos copyright K.C. Jandér
We can test whether there are fitness costs to not cooperating by forcing wasps to cheat by not pollinating, then quantifying the resulting lifetime reproductive success (Nefdt 1989; Jousselin et al. 2003; Jandér and Herre 2010). Previous experiments on actively pollinated fig species found clear fitness reductions for wasps that did not pollinate. These fitness costs were caused by a combination of abortions of unpollinated figs (killing all the wasp larvae), and reduced proportion of wasp larvae that successfully developed to adults (Jandér and Herre 2010; Jansen-González et al. 2012; Jandér and Herre 2016; Dunn 2020). Importantly, we then investigated the corresponding wasp species and found that uncooperative pollinator individuals were more common in fig species with low fitness costs for cheating, ie weak host sanctions (Jandér and Herre 2010). Previously this information only existed for four species-pairs of figs and their pollinator wasps (Jandér and Herre 2010). Here I report results from experiments quantifying the fitness effects for wasps that do not pollinate Panamanian Ficus perforata (Croat 1978), a fifth actively pollinated Neotropical fig species. I then test whether the proportion of uncooperative pollinators is correlated with the resulting sanction strength across all five species-pairs. The new data point is in line with the previous, supporting the hypothesis that host sanctions promote cooperation.
I additionally took the opportunity to do a first minor test of whether sanction strength was related to fruit size or foundress numbers. Host sanctions vary remarkably in strength even across closely related fig species (Jandér and Herre 2010). Strong fig sanctions seem to be very effective at preventing cheating (Jandér and Herre 2010), but presumably having strong sanctions comes with some cost for trees. For example, if it is difficult for the tree to correctly allocate resources according to the degree of pollination (Jandér et al. 2012; Jandér and Herre 2016), there could be costly mistakes. If figs are incorrectly aborted both the investment in the infloresence as well as any potential pollinator offspring and potential seeds are lost. One way for trees to facilitate correct resource allocation could be to partition the crop into many smaller units that each interact with a single foundress wasp. If so, we would expect fig species with small fig size and few foundresses to be able to have stronger sanctions than fig species with large figs and multiple foundresses. The fig species examined here, F. perforata, produces very large crops of among the smallest fig fruits known (Korine et al. 2000; Dunn et al. submitted; Fig. 1), each typically with a single foundress (Herre 1989). This extreme crop partitioning should facilitate for trees to detect poorly pollinated figs, therefore enabling strong sanctions without large risks for mistakes. However, the host sanctions found in small-fruited F. perforata were of intermediate strength. Across five closely related fig species there was no correlation between sanction strength and foundress number or fruit size.
2 Methods
2.1 Study system
The study populations are natural populations of trees and wasps at Barro Colorado Nature Monument (BCNM), Panama. Ficus perforata (Croat 1978), also referred to as Ficus americana subsp. americana: eugeniifolia-form (Berg 2007), belongs to subgenus Urostigma, section Americana, and is a hemiepiphytic strangler Fig. F. perforata carries numerous small fig fruits (on average 30,000 figs per tree, each fig weighs 0.4 g, contains on average 168 flowers, and typically hosts a single foundress wasp (Herre 1989; Korine et al. 2000; Dunn et al. submitted)). The pollinator of F. perforata at BCNM is Pegoscapus insularis sp. A. (Wiebes 1995). Previously there was also another pollinator species of F. perforata at BCNM, Pegoscapus insularis sp. B, but it was rare, and has not been found since 2015 despite extensive screening (E.A. Herre & J.D. Satler personal communication, Satler et al. 2020).
The four fig species studied earlier and included here for comparison are F. citrifolia, F. nymphaeifolia, F. obtusifolia, and F. popenoei (all subgenus Urostigma, section Americana), and their respective pollinators; data from (Jandér and Herre 2010).
2.2 Pollen exclusion experiment
To set up experiments quantifying the fitness outcomes of pollinator wasps that do, or do not, pollinate I matched two trees at different phenological stages: one flowering (receptive), and another producing wasps. Pre-receptive figs on the flowering tree were enclosed in mesh bags to prevent wild wasps from pollinating. Figs from the wasp-producing tree were allocated to two different treatments (Nefdt 1989; Jousselin et al. 2003; Jandér and Herre 2010). In some figs, the pollen-producing male flowers were removed before wasp emergence to prevent wasps from collecting any pollen, i.e. these wasps were forced to become pollen-free (P-). In other figs, wasps were allowed to emerge and collect pollen naturally – these were pollen-carrying (P+) control wasps. A single P+ or P- wasp was then allowed to enter each receptive fig on the flowering tree, producing P+ and P- figs. All experimental figs were of similar size. Mesh bags were replaced and figs were allowed to mature on the tree. Such experiments were set up on two different trees (tree IDs BN5 and BCI12).
Any aborted experimental figs were collected and dissected to verify treatment success. Mature figs were collected just before wasps emerged from the figs, then dissected under a stereomicroscope and the number of seeds, bladders (empty failed galls representing wasp larvae that died partway through development), and male and female wasps were counted for each fig. In a few figs some wasps had escaped before collection – in those cases the number of wasp galls was counted to indicate the total number of pollinator wasps produced in the fig. This is accurate because there were only pollinators and no parasitic wasps in these experimental figs. Figs where the treatment had failed due an incorrect number of foundresses entering the fig (for example 0 or 2 instead of the desired 1), or where seeds were present where none were expected (in P- figs), were excluded from the analyses. On tree BN5, 6 P+ and 2 P- figs were excluded because of an incorrect number of foundresses, and 15 P- figs because of the presence of seeds. On tree BCI12, 3 P+ and 2 P- figs were excluded because of an incorrect number of foundresses, and 1 P- fig because of the presence of seeds. Figs that were excluded due to failed treatment are not included in the numbers of aborted or matured figs.
To enable comparisons of sanction strength with other fig species I followed the calculations as described in Jandér and Herre (2010): I calculated the relative proportion of figs that matured (MR) in the P- treatment compared to the P+ treatment (MR = MP- /MP+), and the relative number of offspring (OR) in the P- treatment compared to the P+ treatment (OR = OP- /OP+). The relative fitness (WR) of a single foundress P- wasp compared to a P+ wasp is (WR = MR x OR). Sanction strength is calculated as 1-WR.
2.3 Naturally occurring pollen free wasps
Unmanipulated wasps from the natural population of the pollinator species were collected on sticky traps as they arrived to flowering trees. A total of 597 wasps were collected at four different flowering trees (10, 16, 287, 284 wasps at each tree respectively). Wasps were examined under a light microscope to determine presence or absence of pollen grains in their pollen pockets. Some wasps had a few scattered pollen grains on their bodies – if there were no more than 5 pollen grains these wasps were still classified as “pollen free” (in contrast, figs entered by pollen-carrying wasps produce on average 90 seeds or more, meaning that P+ wasps on average carry at least 90 pollen grains, likely more).
2.4 Statistical analyses
The proportion of aborted figs were compared with Fisher exact tests. The number of seeds were compared with Mann-Whitney U-tests due to non-normal distributions. The number of wasp offspring were compared with t-tests for equal or unequal variances as appropriate; results did not qualitatively change if I instead used Mann-Whitney U tests. The proportion male wasp offspring per fig was compared using GLM with binomial errors and a logit link. To account for phylogenetic relationships when correlating traits across species I used PGLS, assuming a Brownian motion model of evolution (lambda = 1). Because wasps not carrying pollen is a wasp-characteristic, I based the sanction – pollen free wasp analyses on the most recent fig wasp phylogeny for these species (Satler et al. 2019). For completeness I also repeated the analyses basing them on the most recent Ficus phylogeny for these species (Satler et al. 2019). I based the sanction – fruit mass analyses on the most recent Ficus phylogeny of these species (Satler et al. 2019). Analyses were done using SPSS 27 and R 4.0.
3 Results
3.1 Abortions of figs
On both trees, figs that were entered by a pollen-free wasp (P-) were more likely to be aborted than figs that were entered by a pollen-carrying wasp (P+). Tree BN5 aborted 25 of 61 P- figs and 10 of 53 P+ figs (Fisher exact test, p = 0.009; Fig. 2d). Tree BCI12 aborted 22 of 95 P- figs and 4 of 86 P+ figs (Fisher exact test, p = 0.0003; Fig. 2d). Therefore, the relative proportion of figs that matured in P- figs compared to P+ figs (MR) was 0.73 and 0.81 for tree BN5 and BCI12 respectively.
Pollinator wasps that did not carry pollen (P-) had reduced fitness compared to those that carried pollen (P+) in experiments on two trees (labelled BCI12 and BN5) of Ficus perforata. Not carrying pollen led to (a) decreased seed production, (b) decreased wasp offspring production (in tree BCI12), and (c) increased number of bladders (larvae that failed development). Additionally, (d) a larger proportion of unpollinated (P-) figs compared to pollinated (P+) figs aborted; abortion kills all wasp larvae. Error bars represent 1 sem. Dark grey represents figs entered by a P+ foundress, orange represents those entered by a P- foundress
3.2 Number of seeds, wasp offspring and bladders
As expected, the number of seeds was dramatically higher in matured P+ figs than in matured P- figs on both trees; there were no seeds in P- figs at all. On tree BCI12, P+ figs had on average 91.3 (sem 4.8) seeds, whereas P- figs had 0 (sem 0) seeds (Mann-Whitney U test, U = 420, p < 0.001; Fig. 2a). On tree BN5, P+ figs had on average 103.6 (sem 3.0) seeds, whereas P- figs had 0 (sem 0) seeds (Mann-Whitney U test, U = 450, p < 0.001; Fig. 2a).
On one of the trees, the number of wasp offspring was reduced for P- wasps compared to P+ wasps, but on the other tree it was not. On tree BCI12, P- foundresses had on average 32.2 (sem 1.4) offspring, compared to P+ foundresses 60.2 (sem 2.8) offspring (t-test unequal variances, t29.30 = −8.97, p = 6.8E-10; Fig. 2b). On tree BN5, P- foundresses had on average 77.9 (sem 4.1) offspring, compared to P+ foundresses 74.9 (sem 3.0) offspring (t-test, t43 = 0.59, p = 0.59; Fig. 2b). The relative number of wasp offspring emerging from P- figs compared to P+ figs (OR) was therefore 0.53 and 1.04 for trees BCI12 and BN5 respectively. The proportion male offspring did not significantly differ between the two treatments (Tree BCI12: P- mean 0.075 sem 0.011, P+ mean 0.095 sem 0.006, GLM X239 = 3.64, p = 0.06; Tree BN5: P- mean 0.093 sem 0.009, P+ mean 0.096 sem 0.005, GLM X243 = 1.045, p = 0.31).
On both trees P- figs had a higher number of bladders (galls where wasp larvae died part-way through development) than did P+ figs. On tree BCI12, the average number of bladders in P- figs was 47.55 (sem 1.5) , compared to 11.8 (sem 1.7) in P+ figs (t-test, t39 = 15.82, p = 1.5E-18; Fig. 2c). On tree BN5, the average number of bladders in P- figs was 8.4 (sem 0.8) , compared to 3.2 (sem 0.3) in P+ figs (t-test, t43 = 6.92, p = 1.7E-8; Fig. 2c).
3.3 Sanction strength
To enable comparisons to other studies I calculated sanction strength (1 – the relative fitness of P- compared to P+ wasps) as detailed in (Jandér and Herre 2010). The relative fitness (WR) for a P- wasp compared to a P+ wasp was for BCI12 0.43 and tree BN5 0.76, making the mean WR 0.59, and the mean sanction strength, (1-WR), for F. perforata 0.41.
3.4 Fruit size, foundress number, and sanction strength
Across the five fig species, there was no relationship between the mean dry weight of mature fig fruits (Herre 1989) and sanction strength (Pearson correlation, r = −0.03, p = 0.96; Fig. S1a, Online Resource 1). Controlling for phylogenetic dependencies does not change the result (PGLS, n = 5, t = −0.41, p = 0.71). There was also no relationship between the mean number of foundresses per fig and sanction strength (Pearson correlation, r = −0.06, p = 0.93; Fig. S1b, Online Resource 1). Controlling for phylogenetic dependencies does not change the result (PGLS, n = 5, t = −0.09, p = 0.93).
3.5 Sanction strength and proportion pollen-free wasps
Of the pollinator wasps that arrived at the four flowering F. perforata trees, on average 1.9% (sem 1.5) of wasps carried fewer than five pollen grains in their pollen pockets or scattered on their bodies. Combining this with previously collected data from an additional four species pairs of closely related Ficus (section Urostigma Americana) and Pegoscapus wasps (Jandér and Herre 2010) there was a significant negative correlation between sanction strength and the proportion pollen-free wasps across these five species pairs (Pearson correlation on log-transformed data, r = −0.977, p = 0.004; Fig. 3). The relationship remains significant when controlling for phylogenetic dependencies, estimating the effect of sanction strength on the proportion pollen free wasps to be 0.69 (PGLS using fig wasp phylogeny, n = 5, t = −4.89, p = 0.016; PGLS using Ficus phylogeny, n = 5, t = −9.62, p = 0.002). This pattern corresponds to what we would expect if host sanctions promote cooperation.
4 Discussion
In Panamanian F. perforata there are clear fitness costs for wasps that do not pollinate. These fitness costs are caused by a combination of increased abortions of unpollinated figs, and of fewer maturing wasp offspring in unpollinated figs compared to in pollinated figs. The reduced number of offspring in unpollinated figs is likely caused by a higher mortality of the developing larvae, indicated by the increased number of bladders in these figs (Jandér and Herre 2016). Although I did not test for it here, in other Neotropical actively pollinating fig wasp species there is additionally a size reduction in offspring when developing in unpollinated figs compared to pollinated figs, which additionally lowers fitness (Jandér et al. 2016). All these three components of fitness reduction (abortions, reduced offspring number, reduced offspring size), are compatible with the notion that the fitness reduction is caused by a reduced allocation of resources to unpollinated figs compared to pollinated figs (Jandér and Herre 2016). An alternative explanation of the reduction in number and size of offspring is that larval development is impaired when feeding on unfertilized endosperm compared to fertilized endosperm (Jansen-González et al. 2012). Irrespective of the underlying mechanism, wasps that do not pollinate suffer fitness costs.
The increased abortion and reduced offspring numbers in F. perforata are qualitatively consistent with what has been reported in other actively pollinated fig mutualisms in the Neotropics (Jandér and Herre 2010; Jandér et al. 2012; Jansen-González et al. 2012; Jandér et al. 2016), and worldwide (Nefdt 1989; Jousselin et al. 2003; reviewed in Dunn 2020). However, the degree of fitness costs (host sanction strength) varies dramatically even within this Neotropical subsection (Urostigma Americana) of closely related fig species (Jandér and Herre 2010; Satler et al. 2019). For example, Ficus citrifolia has extremely strong sanctions (0.999), whereas F. popenoei has considerably weaker sanctions (0.33) (Jandér and Herre 2010). The sanction strength of F. perforata (0.41) is intermediate to weak for this neotropical section of figs, and also compared to old world actively pollinated fig species (Jandér and Herre 2010).
The relatively weak host sanctions of F. perforata was unexpected given the small size of its fig fruits. Having the fruit production partitioned into numerous small fruits with few foundresses (such as F. perforata or F. citrifolia) rather than fewer large fruits with several foundresses each (such as F. nymphaeifolia or F. popeneoi), should facilitate for the tree to detect poorly pollinated fig fruits and allocate resources accordingly. I had therefore expected sanction strength to be high in the small-fruited F. perforata, but that was not the case. Across five closely related fig species there was no correlation between sanction strength and fruit size or foundress numbers. Additional factors must be important for determining the strength of host sanctions.
An alternative factor that may affect sanction strength could be pollination levels. If trees frequently lack pollinators due to fragmentation, habitat loss, edge of range, or other reasons, trees may invest in all figs that are pollinated, including poorly pollinated figs, which in effect would mean weaker sanctions. F. perforata trees at BCNM frequently lack pollinators, leading to abortion of entire crops, or of large proportion of crops (KCJ personal observation, A. Gomez personal communication 2020). Could this lead to trees having weaker sanctions? Even if it is a solely plastic response in trees, wasps could still respond to the effective sanction levels of trees (Agrawal 2001). Additional factors that could affect optimal and effective sanction strength respectively include the degree to which the pollen-free trait is heritable, whether foundresses can move between figs (increasing effective foundress numbers), and the prevalence of parasitic wasps. Ongoing work addresses the complicated question of why sanction strength differs across Ficus species.
How do these host sanctions affect cooperation levels in the pollinating wasp species? Naturally occurring wasp individuals that did not carry pollen were relatively common in F. perforata: 1.9%. This is the second highest proportion of naturally occurring pollen-free wasps reported for a species in the genus Pegoscapus (Jandér and Herre 2010), although the relatively low number of wasps examined here makes this estimate somewhat uncertain. Nevertheless, the estimate falls remarkably in line with the previously reported species-pairs when correlating the proportion of pollen free wasps with the experimentally determined sanction strength in their respective host species (Fig. 3). The previous finding that fig species with strong host sanctions are associated with low levels of uncooperative wasps (Jandér and Herre 2010) is supported and strengthened by the current study. The pattern corresponds to what we would expect to see if host sanctions promote cooperation.
Further strong support for the idea that host sanctions promote cooperation and prevent cheating comes from a recent study of a Chinese population of F. microcarpa and its associated wasps, where a complete lack of host sanctions has allowed a non-pollinating cheater wasp species to evolve from a pollinator lineage (Zhang et al. 2021). Altogether, there is ever increasing support that host sanctions promote cooperation. Although it is particularly straightforward to study these questions in highly species-specific systems such as the fig wasp – fig tree mutualism, we expect similar general dynamics in other mutualistic systems.
References
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Ågren JA, Davies NG, Foster KR (2019) Enforcement is central to the evolution of cooperation. Ecol Evol 3:1018–1029
Berg CC (1989) Classification and distribution of Ficus. Experientia 45:605–611
Berg CC (2007) Proposals for treating four species complexes in Ficus subgenus Urostigma section Americanae (Moraceae). Blumea 52:295–312
Bull JJ, Rice WR (1991) Distinguishing mechanisms for the evolution of co-operation. J Theor Biol 149:63–74
Croat TB (1978) Flora of Barro Colorado Island. California, Stanford University Press, Stanford
Cruaud A, Rønsted N, Chantarasuwan B, Chou LS, Clement WL, Couloux A, Cousins B, Genson G, Harrison RD, Hanson PE, Hossaert-McKey M, Jabbour-Zahab R, Jousselin E, Kerdelhue C, Kjellberg F, Lopez-Vaamonde C, Peebles J, Peng Y-Q, Pereira RAS, Schramm T, Ubaidillah R, van Noort S, Weiblen GD, Yang DR, Yodpinyanee A, Libeskind-Hadas R, Cook JM, Rasplus J-Y, Savolainen V (2012) An extreme case of plant-insect codiversification: figs and fig-pollinating wasps. Syst Biol 61:1029–1047
Dunn DW (2020) Stability in the fig tree - fig wasp mutualisms: how to be a cooperative fig wasp. Biol J Linn Soc 130:1–17
Dunn, D. W., K. C. Jandér, E. A. Herre, S. al-Beidh, S. T. Segar, D. W. Yu, J. Ridley, D. M. Windsor, H. Yu and J. M. Cook (submitted). Infloresence size predicts flower exploitation by pollinating wasps across fig tree- fig wasp mutualisms
Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S (2017) The evolution of the host microbiome as an ecosystem on a leash. Nature 548:43–51
Frank SA (1984) The behaviour and morphology of the fig wasps Pegoscapus assuetus and P. jimenezi: descriptions and suggested behavioural characters for phylogenetic studies. Psyche 91:289–308
Frederickson ME (2017) Mutualisms are not on the verge of breakdown. Trends Ecol Evol 32:727–734
Herre EA (1989) Coevolution of reproductive characteristics in 12 species of New World figs and their pollinator wasps. Experientia 45:637–647
Herre EA, Jandér KC, Machado CA (2008) Evolutionary ecology of figs and their associates: ongoing progress and outstanding puzzles. Annu Rev Ecol Syst 39:439–458
Jandér KC, Dafoe A, Herre EA (2016) Fitness reduction for uncooperative wasps through reduced offspring size: a third component of host sanctions. Ecology 97:2491–2500
Jandér KC, Herre EA (2010) Host sanctions and pollinator cheating in the fig tree - fig wasp mutualism. Proc Royal Soc London, B Ser 277:1481–1488
Jandér KC, Herre EA (2016) Host sanctions in Panamanian Ficus are likely based on selective resource allocation. Am J Bot 103:1753–1762
Jandér KC, Herre EA, Simms EL (2012) Precision of host sanctions in the fig tree - fig wasp mutualism: consequences for uncooperative symbionts. Ecol Lett 15:1362–1369
Jandér KC, Steidinger BS (2017) Why mutualist partners vary in quality: mutation-selection balance and incentives to cheat in the fig tree - fig wasp mutualism. Ecol Lett 20:922–932
Jansen-González S, Teixeira SP, Pereira AS (2012) Mutualism from the inside: coordinated development of plant and insect in an active pollinating fig wasp. Arthropod Plant Interact 6:601–609
Jousselin E, Hossaert-McKey M, Herre EA, Kjellberg F (2003) Why do fig wasps actively pollinate monoecious figs? Oecologia 134:381–387
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bücking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882
Kiers ET, Rousseau RA, West SA, Denison RF (2003) Host sanctions and the legume-rhizobium mutualism. Nature 425:78–81
Kjellberg F, Jousselin E, Bronstein JL, Patel A, Yokoyama J, Rasplus J-Y (2001) Pollination mode in fig wasps: the predictive power of correlated traits. Proc R Soc Lond Ser B 268:1113–1121
Korine C, Kalko KV, Herre EA (2000) Fruit characteristics and factors affecting fruit removal in a Panamanian community of strangler figs. Oecologia 123:560–568
Machado CA, Robbins N, Gilbert MTP, Herre EA (2005) Critical review of host specificity and its coevolutionary implications in the fig/fig-wasp mutualism. PNAS 102:6558–6565
Nefdt, R. J. C. (1989). Interactions between fig wasps and their host figs PhD thesis, Rhodes University
Ollerton J, Winfree R, Tarrant S (2011) How many flowering plants are pollinated by animals? Oikos 120:321–326
Parniske M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Rev 6:763–775
Pellmyr O, Kjellberg F, Herre EA, Kawakita A, Hembry DH, Holland JN, Terrazas T, Clement WL, Segraves KA, Althoff DM (2020) Active pollination drives selection for reduced pollen-ovule ratios. Am J Bot 107:164–170
Rasplus, J.-Y., L. J. Rodriguez, L. Sauné, Y.-Q. Peng, A. Bain, F. Kjellberg, R. D. Harrison, R. A. S. Pereira, R. Ubaidillah, C. Tollon-Cordet, M. Gautier, J. P. Rossi and A. Cruaud (2020). Exploring systematic biases, rooting methods and morphological evidence to unravel the evolutionary history of the genus Ficus (Moraceae). Cladistics 0: 1–21
Sachs JL, Mueller UG, Wilcox TP, Bull JJ (2004) The evolution of cooperation. Q Rev Biol 79:135–160
Satler JD, Herre EA, Heath TA, Machado CA, Gómez Zúñiga A, Nason JD (2020). Genome-wide sequence data show no evidence of admixture and introgression among pollinator wasps associated with a community of Panamanian strangler figs. BioRxiv. https://doi.org/10.1101/2020.12.09.418376
Satler JD, Herre EA, Jandér KC, Eaton DAR, Machado CA, Heath TA, Nason JD (2019) Inferring processes of coevolutionary diversification in a community of Panamanian strangler figs and associated pollinating wasps. Evolution 73:2295–2311
Shanahan M, So S, Compton SG, Corlett R (2001) Fig-eating by vertebrate frugivores: a global review. Biol Rev 76:529–572
Wang G, Zhang X, Herre EA, McKey D, Machado CA, Yu WB, Cannon CH, Arnold ML, Pereira RAS, Ming R, Liu YF, Wang Y, Ma D, Chen J (2021) Genomic evidence of prevalent hybridization throughout the evolutionary history of the fig-wasp pollination mutualism. Nat Commun 12:718
Wei ZD, Kobmoo N, Cruaud A, Kjellberg F (2014) Genetic structure and hybridization in the species group of Ficus auriculata: can closely related sympatric Ficus species retain their genetic identity while sharing pollinators? Mol Ecol 23:3538–3550
West SA, Griffin AS, Gardner A (2007) Evolutionary explanations for cooperation. Curr Biol 17:R661–R672
Wiebes JT (1995) The New World Agaonidae: pollinators of figs. Proc Koninklijke Nederlandse Akademie van Wetenschappen 98:167–183
Zhang T, Jandér KC, Huang JF, Wang B, Zhao JB, Miao BG, Peng Y-Q, Herre EA (2021) The evolution of parasitism from mutualism in wasps pollinating the fig, Ficus microcarpa, in Yunnan Province, China.PNAS In press
Acknowledgements
I thank Lovisa Dück for help with fieldwork, Brian Steidinger, Allen Herre, and an anonymous reviewer, for helpful comments, the Wenner-Gren Foundations for funding, and the Smithsonian Tropical Research Institute for providing facilities and support during fieldwork.
Availability of data and material
Data is available at Zenodo; DOI: https://doi.org/10.5281/zenodo.5006747.
Code availability
Not applicable.
Funding
Open access funding provided by Uppsala University. Research funding was provided by the Wenner-Gren Foundations.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest/competing interests
There are no conflicts of interest or competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 128 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jandér, K.C. Fitness costs for fig wasps that fail to pollinate their host Ficus perforata. Symbiosis 84, 171–178 (2021). https://doi.org/10.1007/s13199-021-00781-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-021-00781-5