Abstract
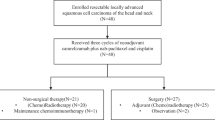
Head and neck squamous cell carcinomas (HNSCC) have proven to be inherently resistant to systemic treatments as a result of histological, molecular, and etiological heterogeneity, with limited responses seen after second-line therapy and beyond. With limited treatment options after progression on systemic chemotherapy in HNSCCs, immunotherapy has a role to play with improved results. In this prospective, observational, non-randomized, open-label study, a total of 12 patients with advanced, relapsed, or metastatic HNSCC received Inj. Nivolumab weight-based dose of 3 mg per kg, intravenously every 2 weeks along with low-dose capecitabine 500 mg twice a day, was prospectively assessed. The patient’s clinical, hematological, and staging characteristics were described and the clinical benefit rate (CBR) was calculated. A total of 12 patients received the combined metronomic chemo-immunotherapy (CMCI). The majority of patients were belonging to ECOG-PS 1(66%), with all patients being in stage IV disease. Six, four, and two patients received immunotherapy as the 5th, 3rd, and 4th line of therapy, respectively. Nivolumab and low-dose capecitabine were used in all 12 patients. CBR was seen in 66% (8/12) of patients, one patient died due to hepatitis and hepatic encephalopathy, another patient died due to pneumonia and respiratory complications, two patients had progressive disease, and two patients with stable disease discontinued treatment because of financial constraints and kept on capecitabine alone. The majority tolerated therapy well with no grade 3/4 immune-related adverse events (IRAEs). Two patients required supportive therapy with packed red cell transfusion and albumin infusions. Six-month overall survival (OS) and progression-free survival (PFS) in the study population were 83.3% and 66.6%, respectively. In conclusion, nivolumab along with metronomic chemotherapy with low-dose capecitabine was very well tolerated and exhibited anti-tumor activity with a CBR of 66%, 6-month OS of 83.3%, and 6-month PFS of 66.6%, in extensively pretreated patients with HNSCCs. Additional studies of nivolumab and metronomic chemotherapy and immuno-immuno combination therapy in these diseases are ongoing.


Similar content being viewed by others
Data Availability
All required data is available in the article itself, so if any updates are available we will provide as and when required.
Abbreviations
- ECOG:
-
Eastern Cooperative Oncology Group
- Ca BM:
-
Carcinoma Buccal Mucosa
- Ca BOT:
-
Carcinoma of Base of Tongue
- SqCC:
-
Squamous cell carcinoma
- PCV:
-
Packed cell volume
- Alb:
-
Albumin
- PD:
-
Progressive disease
- PR:
-
Partial response
- LFU:
-
Lost to follow-up
- SD:
-
Stable disease
References
Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F et al (2017) GBD 2016 Disease and injury incidence and prevalence collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100):1211–59
Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C (2015) Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 33(29):3235–3242
Argiris A, Li S, Ghebremichael M, Egloff AM, Wang L, Forastiere AA et al (2014) Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: an analysis of Eastern Cooperative Oncology Group trials. Ann Oncol 25(7):1410–1416
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359(11):1116–1127
Zang X (2018) 2018 Nobel Prize in medicine awarded to cancer immunotherapy: Immune checkpoint blockade – a personal account. Genes Dis 5(4):302–303
Sundar R, Cho B, Brahmer JR, Soo AR (2015) Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol 7(2):85–96
Bellone S, Buza N, Choi J, Zammataro L, Gay L, Elvin J et al (2018) Exceptional response to pembrolizumab in a metastatic, chemotherapy/radiation-resistant ovarian cancer patient harboring a PD-L1-genetic rearrangement. Clin Cancer Res 24(14):3282–3291
Facchinetti F, Bordi P, Leonetti A, Buti S, Tiseo M (2018) Profile of atezolizumab in the treatment of metastatic non-small-cell lung cancer: patient selection and perspectives. Drug Des Devel Ther 12:2857–2873
Onyshchenko M (2018) The puzzle of predicting response to immune checkpoint blockade. EBioMedicine 33:18–19
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10(9):909–915
Bailly C, Thuru X, Quesnel B (2020) Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer 2(1):zcaa002. https://doi.org/10.1093/narcan/zcaa002
Revannasiddaiah S, Madabhavi I, Bodh A, Thakur P, Sharma M (2015) Metronomic chemotherapy in anaplastic thyroid carcinoma: a potentially feasible alternative to therapeutic nihilism. Indian J Palliat Care 21(2):245–249
Revannasiddaiah S, Pandey KC, Madabhavi IV et al (2019) Evaluation of continuous low dose versus standard dose capecitabine monotherapy as second/third-line chemotherapy for metastatic malignancies. Ann Oncol 30(suppl_9):ix118–ix121. https://doi.org/10.1093/annonc/mdz430
Madabhavi IV, Sarkar M, Sagar R (2021) Metronomic low-dose capecitabine in metastatic, recurrent, or persistent carcinoma of the cervix as 3rd line and beyond. Ann Oncol 32(suppl_5):S725–S772. https://doi.org/10.1016/annonc/annonc703
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 359(11):1116–27
Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348:56–61
Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27:450–461
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375(19):1856–1867
Zandberg DP, Strome SE (2014) The role of the PD-L1: PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol 50(7):627–632
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028
Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M et al (2017) Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and checkMate 057). J Clin Oncol 35:3924–3933
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092
Cramer JD, Burtness B, Ferris RL (2019) Immunotherapy for head and neck cancer: recent advances and future directions. Oral Oncol 99:104460
Gupta VG, Bakhshi S (2017) Pediatric hematopoietic stem cell transplantation in India: status, challenges and the way forward: Based on Dr. K. C. Chaudhuri oration 2016. Indian J Pediatr 84:36–41
Maiti R (2014) Metronomic chemotherapy. J Pharmacol Pharmacother 5(3):186–192
Nai-Wen Su, Chen Y-J (2021) Metronomic therapy in oral squamous cell carcinoma. J Clin Med 10(13):2818
Patil Vijay Maruti, Noronha Vanita, Menon Nandini, Rai Rahul, Bhattacharjee Atanu, Singh Ajay et al (2023) Low-dose immunotherapy in head and neck cancer: a randomized study. J Clin Oncol 41(2):222–23
Iqbal H (2016) Quintin Pan; Capecitabine for treating head and neck cancer. Expert Opin Investig Drugs 25(7):851–859
Van Der Kraak L, Goel G, Ramanan K, Kaltenmeier C, Zhang L, Normolle DP et al (2016) 5-Fluorouracil up-regulates cell surface B7–H1 (PD-L1) expression in gastrointestinal cancer. J Immunother Cancer 4:65
Chen YL, Chang MC, Cheng WF (2017) Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett 400:282–292
Kareva I (2017) A combination of immune checkpoint inhibition with metronomic chemotherapy as a way of targeting therapy-resistant cancer cells. Int J Mol Sci 18:2134
Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD et al (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162:1229–1241
Mpekris F, Voutouri C, Panagi M, Baish JW, Jain RK, Stylianopoulos T (2022) Normalizing tumor microenvironment with nanomedicine and metronomic therapy to improve immunotherapy. J Control Release 345:190–199
Aravind S, Jose J, Shenoy PK, Avaronnan M, Thavarool SB, Nayanar SK (2022) The spectrum of histomorphological changes and pathological tumor response following preoperative oral metronomic chemotherapy in oral squamous cell carcinoma South Asian J. Cancer 11(2):146–151
Martinez-Trufero J, Isla D, Adansa J, Irigoyen A, Hitt R, Gil-Arnaiz I et al (2010) Phase II study of capecitabine as palliative treatment for patients with recurrent and metastatic squamous head and neck cancer after previous platinum-based treatment. Br J Cancer 102:1687–1691
Wu Y, Deng Z, Wang H, Ma W, Zhou C, Zhang S (2016) Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol 17:29
Author information
Authors and Affiliations
Contributions
IM conceived and designed the experiment, made critical revisions, and approved the final version. IM, MS, VK, and RS analyzed the data and wrote the first draft of the manuscript. IM, MS, VK, and RS contributed to the writing of the manuscript. IM, MS, VK, and RS agree with the manuscript results and conclusions. IM, MS, VK, and RS jointly developed the structure and arguments for the paper. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
None declared. Initial 2-year data has been accepted in an abstract form in the Proceedings of the AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics; 2021 Oct 7–10. Philadelphia (PA): AACR; Mol Cancer Ther 2021;20(12 Suppl): Abstract nr P101.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Madabhavi, I., Sarkar, M., Kumar, V. et al. Combined Metronomic Chemo-immunotherapy (CMCI) in Head and Neck Cancers–An Experience from a Developing Country. Indian J Surg Oncol (2024). https://doi.org/10.1007/s13193-024-01900-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13193-024-01900-6