Abstract
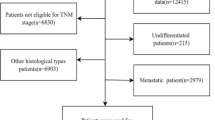
Neoadjuvant chemotherapy (NACT) for locally advanced breast cancer (LABC), apart from increasing breast conservation rates, also provides an opportunity to assess tumour response to chemotherapy, with Pathological Complete Response (pCR) described as an independent prognostic factor and a surrogate marker for better outcome and survival. Our primary aim was to identify clinical and pathological factors associated with pCR following NACT in patients with LABC treated at our institution. Our secondary aim was to analyze the impact of pCR and associated factors on disease free survival (DFS) and overall survival (OS). A retrospective analysis of LABC patients treated with NACT between Jun 2011 and Dec 2013. Clinical and histological variables were analyzed for association with pCR (no invasive or in situ carcinoma in breast or axillary lymph nodes). Kaplan-Meier curves and Cox regression model was used for survival analysis. All values were twosided, and statistical significance was defined as p < 0.05. 240 patients were included. The median tumor size was 6 cm, with T4 disease in 49.8 %. 45 % of tumors were of low grade (G1 + G2) and 53.8 % of high grade (G3). Estrogen Receptor (ER) was positive in 70.8 %, progesterone receptor (PR) in 53.3 % and Her2 in 38.8 %. The preferred NACT regimen was sequential anthracycline and taxane and 88.8 % of patients received this regimen. Of 93 potential Her2 Positive patients, only 23 received trastuzumab. Overall 23.2 % patients had pCR. At median follow up of 21 months (range, 3–42), 16.3 % of patients had recurrent disease, and 6.7 % had died. High tumor grade (p = 0.04), PR negative status (p < 0.01) and trastuzumab treatment (p = 0.01) were significant predictors of pCR in univariate analysis. On multivariate analysis PR negativity (OR 3.2, 95 % CI = 1.6 to 6.04, p = 0.001) and Trastuzumab use (OR 0.24, 95 % CI = 0.1 to 0.6, p = 0.004) were significant. Patients with pCR had positive associations with survival (p < .02,OS& .02,DFS) and interestingly PR positivity had positive association with DFS (p = 0.02) in Kaplan-Meier curves. On Cox regression, PR positivity (HR = 0.3, p < 0.01) and pCR (HR = 0.2, p < 0.01) correlated with DFS, though not with early OS. for the PR positive patients were paradoxical. Though less likely to have pCR (15 %, vs 32 % if PR negative), they had better DFS (p = 0.02), and achieving pCR had no survival benefit in this group. In contrast, PR negative patients, irrespective of ER status, had a high pCR rate, and achieving pCR had survival advantage (p < 0.05,DFS& p < 0.02,OS). PR negative patients without pCR had the worst DFS (p < 0.01) among all. High grade and Trastuzumab treatment as predictors of pCR, and pCR as a surrogate marker for survival are well recognized, and are supported by our findings. In present cohort, PR negativity showed prognostic importance independent of ER status. However these results were derived from sub-group, post-hoc analysis of data from a pre-existing cohort, without ‘a-priori’ hypothesis for survival analysis in relation to PR. These “hypothesis generating” results need confirmation by a well-designed prospective cohort or a randomized trial.



Similar content being viewed by others
References
Tewari M, Krishnamurthy A, Shukla HS (2008) Predictive markers of response to neoadjuvant chemotherapy in breast cancer. Surg Oncol. 17:301–311
Colleoni M, Zahrieh D, Gelber RD, Viale G, Luini A, Veronesi P, et al. (2003) Preoperative systemic treatment: prediction of responsiveness. Breast 12:538–542
Guarneri V, Broglio K, Kau SW, et al. (2006) Prognostic value of pathological complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol 24:1037–1044
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N (2008) Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and bowel project protocols B-18 and B-27. J Clin Oncol 26:778–785
Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, et al. (2004) Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol 22:2303–2312
Hammond MEH, Hayes DF, Wolff AC, Mangu PB, Temin S (2010) American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Journal of Oncology Practice 6(4):195–197
Wolff AC, Hammond MEH, Hicks DG, et al. (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
Tavassoli FA, Devilee P (2003) World Health Organization classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. IARC Press, Lyon
Von Minckwitz G, Untch M, Blohmer JU, et al. (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–804.24
Carey LA, Dees EC, Sawyer L, et al. (2007) The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13(8):2329–2334
Cortazar P, Zhang L, Untch M, et al. (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
Fumagalli D, Bedard PL, Nahleh Z, et al. (2012) A common language in neoadjuvant breast cancer clinical trials: proposals for standard definitions and endpoints. Lancet Oncol 13:e240–e248. doi:10.1016/S1470-2045(11)70378-3
Kim MM, Allen P, Gonzalez-Angulo AM, et al. (2013) Pathologic complete response to neoadjuvant chemotherapy with trastuzumab predicts for improved survival in women with HER2-overexpressing breast cancer. Ann Oncol 24(8):1999–2004
Peintinger F, Buzdar AU, Kuerer HM, et al. (2008) Hormone receptor status and pathologic response of HER2-positive breast cancer treated with neoadjuvant chemotherapy and trastuzumab. Ann Oncology 19(12):2020–2025
E. H. Lips, L. Mulder ,J. J. de Ronde ,I. A. M. Mandjes ,B. B. Koolen, L. F. A. Wessels, S. Rodenhuis, J. Wesseling. Breast cancer subtyping by immunohistochemistry and histological grade outperforms breast cancer intrinsic subtypes in predicting neoadjuvant chemotherapy response, Breast Cancer Res Treat 2013 140:63–71
J. M. Perez Garcia, C. Saura, E. Muñoz, G. Sanchez-Olle, P. Gomez, V. Peg, D. Sabadell, J. Cortes, J. Baselga, M. Bellet. (2009) Role of progesterone receptor status (PR) as predictive factor of pathological complete response (pCR) to neoadjuvant chemotherapy (NACT) in breast cancer (BC) patients (pts). J Clin Oncol 27:15 s, (suppl; abstr 637)
Baselga J, Bradbury I, Eidtmann H, et al. (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 18:633–640
Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J¨r, Eidtmann H, Gerber B, Hanusch C, Ku¨hn T, du Bois A, Blohmer J-U, Thomssen C, Costa SD, Jackisch C, Kaufmann M, Mehta K, von Minckwitz G (2010) Neoadjuvant TreatmentWith trastuzumab in HER2-positive breast cancer: results from the Gepar Quattro study. J Clin Oncol 28(12):2024–2031
Goldhirsch A, Wood WC, Coates AS, et al. (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol 22:1736–1747. doi:10.1093/annonc/mdr304
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1):25–32. doi:10.1016/S1470-2045(11)70336-9
Nishimukai A, Yagi T, Yanai A, Miyagawa Y, Enomoto Y, Murase K, Imamura M, Takatsuka Y, Sakita I, Hatada T, Miyoshi Y. (2014) High Ki-67 Expression and Low Progesterone Receptor Expression Could Independently Lead to a Worse Prognosis for Postmenopausal Patients With Estrogen Receptor-Positive and HER2-Negative Breast Cancer, Clin breast cancer Dec 24. pii: S1526–8209(14)00288–2. doi: 10.1016/j.clbc.2014.12.007.
G. Cancello, P. Maisonneuve,, N. Rotmensz, G. Viale, M. Colleon, Progesterone receptor loss identifies Luminal B breast cancer subgroups at higher risk of relapse: Ann Oncol, 2013:661–668
Purdie CA, Quinlan P, Jordan LB, Ashfield A, Ogston S, Dewar JA, Thompson AM (2014) Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. British Journal of Cancer 110:565–572. doi:10.1038/bjc.2013.756
Burstein HJ (2010) Ann Alexis Prestrud, Jerome Seidenfeld, Holly Anderson, Thomas a. Buchholz, Nancy E. Davidson, Karen E. Gelmon et al. “American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer.”Journal of. Clin Oncol JCO-2009
Lamy P-J, Pujol P, Thezenas S, Kramar A, Guilleux PRF, Grenier J (2002) Progesterone receptor quantification as a strong prognostic determinant in postmenopausal breast cancer women under tamoxifen therapy. Breast Cancer Res Treat 76:65–71
Clark GM, McGuire WL, Hubay CA, et al. (1983) Progesterone receptor as a prognostic factor instage II breast cancer. N Engi J Med 309:1343–1347
Daniel AR, Hagan CR, Lange CA (2011) Progesterone receptor action: defining a role in breast cancer. Expert Rev Endocrinol Metab 6(3):359–369. doi:10.1586/eem.11.25
Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB (2002) Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 277:5209–5218
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomize trials. Lancet. 2005; 365:1687–1717. doi:10.1016/S0140–6736(05)66544–0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors have no conflict of interests.
Rights and permissions
About this article
Cite this article
Agrawal, S., Banswal, L., Saha, A. et al. Progesterone Receptors, Pathological Complete Response and Early Outcome for Locally Advanced Breast Cancer – a Single Centre Study. (PPLB – 01). Indian J Surg Oncol 7, 397–406 (2016). https://doi.org/10.1007/s13193-016-0523-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-016-0523-3