Abstract
Background
The annually recorded incidence of primary hepatic carcinomas has significantly increased over the past two decades accounting for over 800 thousand of annual deaths caused by hepatocellular carcinoma (HCC) alone globally. Further, secondary liver malignancies are much more widespread compared to primary hepatic carcinomas: almost all solid malignancies are able to metastasise into the liver. The primary tumours most frequently metastasising to the liver are breast followed by colorectal carcinomas. Given the increased incidence of both primary and metastatic liver cancers, a new, revised approach is needed to advance medical care based on predictive diagnostics, innovative screening programmes, targeted preventive measures, and patient stratification for treatment algorithms tailored to individualised patient profile.
Advantages of the approach taken
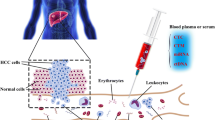
The current pilot study took advantage of systemic alterations characteristic for liver malignancies, utilising liquid biopsy (blood samples) and specific biomarker patterns detected. Key molecular pathways relevant for pathomechanisms of liver cancers have been considered opening a perspective for both—individualised diagnostics and targeted treatment. Systemic alterations have been analysed prior to the therapy application avoiding molecular biological effects potentially diminishing predictive power of the biomarker-panel proposed. Multi-omics at DNA and protein (both expression and activity) levels has been applied. An established biomarker panel is considered as a powerful tool for individualised patient profiling and improved multi-level diagnostics—both predictive and prognostic ones.
Results and conclusions
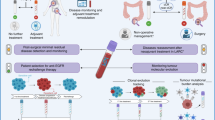
Biomarker panels have been created for the patient stratification, prediction of a more optimal therapy and prognosis of survival based on the individualised patient profiling. Although there are some limitations of the pilot study performed, the results are encouraging, as it may be possible, through further research along these lines, to find a clinically and cost-effective means of stratifying liver cancer patients for personalised care and therapy. The benefits to the patient and society of accurate treatment stratification cannot be overemphasised.



Similar content being viewed by others
Abbreviations
- HCC:
-
hepatocellular carcinoma
- SIRT:
-
selective internal radiation therapy
- TACE:
-
transarterial chemoembolisation
- OS:
-
overall survival
- MMP:
-
metalloproteinase
- SOD-2:
-
superoxide-dismutase 2
- PPPM:
-
predictive preventive personalised medicine
References
Ananthakrishnan A, Gogineni V, Saeian K. Epidemiology of primary and secondary liver cancers. Semin Interv Radiol. 2006;23:47–63. https://doi.org/10.1055/s-2006-939841.
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–27. https://doi.org/10.1001/jamaoncol.2015.0735.
Duarte G, Williams CJ, Vasconcelos P, Nogueira P. Capacity to report on mortality attributable to chronic hepatitis B and C infections by member states: an exercise to monitor progress towards viral hepatitis elimination. J Viral Hepat. 2018;25:878–82. https://doi.org/10.1111/jvh.12882.
Golubnitschaja O, Sridhar KC. Liver metastatic disease: new concepts and biomarker panels to improve individual outcomes. Clin Exp Metastasis. 2016;33:743–55. https://doi.org/10.1007/s10585-016-9816-8.
Ayoub JP, Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL. Unknown primary tumors metastatic to liver. J Clin Oncol. 1998;16:2105–12. https://doi.org/10.1200/JCO.1998.16.6.2105.
Mokdad AA, Singal AG, Yopp AC. Advances in local and systemic therapies for hepatocellular cancer. Curr Oncol Rep. 2016;18:9. https://doi.org/10.1007/s11912-015-0494-5.
Grech G, Zhan X, Yoo BC, Bubnov R, Hagan S, Danesi R, et al. EPMA position paper in cancer: current overview and future perspectives. EPMA J. 2015;6:9. https://doi.org/10.1186/s13167-015-0030-6.
Golubnitschaja O, Yeghiazaryan K, Stricker H, Trog D, Schild HH, Berliner L. Patients with hepatic breast cancer metastases demonstrate highly specific profiles of matrix metalloproteinases MMP-2 and MMP-9 after SIRT treatment as compared to other primary and secondary liver tumours. BMC Cancer. 2016;16:357. https://doi.org/10.1186/s12885-016-2382-2.
Hong Y-F, Chen Z-H, Wei L, Ma X-K, Li X, Wen J-Y, et al. Identification of the prognostic value of lymphocyte-to-monocyte ratio in patients with HBV-associated advanced hepatocellular carcinoma. Oncol Lett. 2017;14:2089–96. https://doi.org/10.3892/ol.2017.6420.
Shen H, Zheng S, Chen R, Jin X, Xu X, Jing C, et al. Prognostic significance of serum procalcitonin in patients with unresectable hepatocellular carcinoma treated with transcatheter arterial chemoembolization: a retrospective analysis of 509 cases. Medicine (Baltimore). 2017;96:e7438. https://doi.org/10.1097/MD.0000000000007438.
Ichikawa T, Machida N, Sasaki H, Tenmoku A, Kaneko H, Negishi R, et al. Early prediction of the outcome using tumor markers and mRECIST in unresectable hepatocellular carcinoma patients who underwent transarterial chemoembolization. Oncology. 2016;91:317–30. https://doi.org/10.1159/000448999.
Soydal C, Keskin O, Kucuk ON, Ozkan E, Bilgic S, Idilman R, et al. Prognostic factors for prediction of survival of hepatocellular cancer patients after selective internal radiation therapy. Ann Nucl Med. 2015;29:426–30. https://doi.org/10.1007/s12149-015-0962-x.
Kim SS, Cho HJ, Won JH, Bae JI, Kang DR, Lee J-D, et al. Interleukin-8 level as a prognostic marker in patients with hepatitis B virus-associated hepatocellular carcinoma treated with transarterial chemoembolization. Cytokine. 2015;76:449–57. https://doi.org/10.1016/j.cyto.2015.07.001.
Zhang X, Long Q. Elevated serum plasma fibrinogen is associated with advanced tumor stage and poor survival in hepatocellular carcinoma patients. Medicine (Baltimore). 2017;96:e6694. https://doi.org/10.1097/MD.0000000000006694.
Ma X-L, Jiang M, Zhao Y, Wang B-L, Shen M-N, Zhou Y, et al. Application of serum Annexin A3 in diagnosis, outcome prediction and therapeutic response evaluation for patients with hepatocellular carcinoma. Ann Surg Oncol. 2018;25:1686–94. https://doi.org/10.1245/s10434-018-6402-0.
Margaritelis NV, Veskoukis AS, Paschalis V, Vrabas IS, Dipla K, Zafeiridis A, et al. Blood reflects tissue oxidative stress: a systematic review. Biomarkers 2015;20:97–108. https://doi.org/10.3109/1354750X.2014.1002807.
Coriat R, Nicco C, Chéreau C, Mir O, Alexandre J, Ropert S, et al. Sorafenib-induced hepatocellular carcinoma cell death depends on reactive oxygen species production in vitro and in vivo. Mol Cancer Ther. 2012;11:2284–93. https://doi.org/10.1158/1535-7163.MCT-12-0093.
Marra M, Sordelli IM, Lombardi A, Lamberti M, Tarantino L, Giudice A, et al. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: an overview. J Transl Med. 2011;9:171. https://doi.org/10.1186/1479-5876-9-171.
Teppo H-R, Soini Y, Karihtala P. Reactive oxygen species-mediated mechanisms of action of targeted cancer therapy. Oxid Med Cell Longev. 2017;2017:1485283. https://doi.org/10.1155/2017/1485283.
Yang S-F, Chang C-W, Wei R-J, Shiue Y-L, Wang S-N, Yeh Y-T. Involvement of DNA damage response pathways in hepatocellular carcinoma. Biomed Res Int. 2014;2014:153867. https://doi.org/10.1155/2014/153867.
Nishida N, Kudo M. Oxidative stress and epigenetic instability in human hepatocarcinogenesis. Dig Dis. 2013;31:447–53. https://doi.org/10.1159/000355243.
Mansoori AA, Jain SK. Molecular links between alcohol and tobacco induced DNA damage, gene polymorphisms and patho-physiological consequences: a systematic review of hepatic carcinogenesis. Asian Pac J Cancer Prev. 2015;16:4803–12.
Miller P, Kidwell KM, Thomas D, Sabel M, Rae JM, Hayes DF, et al. Elevated S100A8 protein expression in breast cancer cells and breast tumor stroma is prognostic of poor disease outcome. Breast Cancer Res Treat. 2017;166:85–94. https://doi.org/10.1007/s10549-017-4366-6.
Zhong J-M, Li J, Kang A-D, Huang S-Q, Liu W-B, Zhang Y, et al. Protein S100-A8: a potential metastasis-associated protein for breast cancer determined via iTRAQ quantitative proteomic and clinicopathological analysis. Oncol Lett. 2018;15:5285–93. https://doi.org/10.3892/ol.2018.7958.
Moz S, Basso D, Bozzato D, Galozzi P, Navaglia F, Negm OH, et al. SMAD4 loss enables EGF, TGFβ1 and S100A8/A9 induced activation of critical pathways to invasion in human pancreatic adenocarcinoma cells. Oncotarget. 2016;7:69927–44. https://doi.org/10.18632/oncotarget.12068.
De Ponti A, Wiechert L, Schneller D, Pusterla T, Longerich T, Hogg N, et al. A pro-tumorigenic function of S100A8/A9 in carcinogen-induced hepatocellular carcinoma. Cancer Lett. 2015;369:396–404. https://doi.org/10.1016/j.canlet.2015.09.005.
Németh J, Stein I, Haag D, Riehl A, Longerich T, Horwitz E, et al. S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology 2009;50(4):1251–62. https://doi.org/10.1002/hep.23099.
Golubnitschaja-Labudova O, Liu R, Decker C, Zhu P, Haefliger IO, Flammer J. Altered gene expression in lymphocytes of patients with normal-tension glaucoma. Curr Eye Res. 2000;21:867–76.
Golubnitschaja O, Filep N, Yeghiazaryan K, Blom HJ, Hofmann-Apitius M, Kuhn W. Multi-omic approach decodes paradoxes of the triple-negative breast cancer: lessons for predictive, preventive and personalised medicine. Amino Acids. 2018;50:383–95. https://doi.org/10.1007/s00726-017-2524-0.
Golubnitschaja O, Yeghiazaryan K, Abraham J-A, Schild HH, Costigliola V, Debald M, et al. Breast cancer risk assessment: a non-invasive multiparametric approach to stratify patients by MMP-9 serum activity and RhoA expression patterns in circulating leucocytes. Amino Acids. 2017;49:273–81. https://doi.org/10.1007/s00726-016-2357-2.
Golubnitschaja O, Moenkemann H, Kim K, Mozaffari MS. DNA damage and expression of checkpoint genes p21(WAF1/CIP1) and 14-3-3 sigma in taurine-deficient cardiomyocytes. Biochem Pharmacol. 2003;66:511–7.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. https://doi.org/10.3322/caac.21338.
Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. https://doi.org/10.4103/jcar.JCar_9_16.
Piñero F, Pages J, Marciano S, Fernández N, Silva J, Anders M, et al. Fatty liver disease, an emerging etiology of hepatocellular carcinoma in Argentina. World J Hepatol. 2018;10:41–50. https://doi.org/10.4254/wjh.v10.i1.41.
Stewart B, Wild C. World Cancer Report 2014 [Internet]. [cited 2018 May 28]. Available from: http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014
Golubnitschaja O, Debald M, Yeghiazaryan K, Kuhn W, Pešta M, Costigliola V, et al. Breast cancer epidemic in the early twenty-first century: evaluation of risk factors, cumulative questionnaires and recommendations for preventive measures. Tumour Biol 2016;37:12941–57. https://doi.org/10.1007/s13277-016-5168-x.
Vaz-Luis I, Lin NU, Keating NL, Barry WT, Winer EP, Freedman RA. Factors associated with early mortality among patients with de novo metastatic breast cancer: a population-based study. Oncologist. 2017;22:386–93. https://doi.org/10.1634/theoncologist.2016-0369.
Polivka J, Kralickova M, Polivka J, Kaiser C, Kuhn W, Golubnitschaja O. Mystery of the brain metastatic disease in breast cancer patients: improved patient stratification, disease prediction and targeted prevention on the horizon? EPMA J. 2017;8:119–27. https://doi.org/10.1007/s13167-017-0087-5.
Golubnitschaja O. Feeling cold and other underestimated symptoms in breast cancer: anecdotes or individual profiles for advanced patient stratification? EPMA J. 2017;8:17–22. https://doi.org/10.1007/s13167-017-0086-6.
Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. https://doi.org/10.1186/s13167-016-0072-4.
Arai K, Takano S, Teratani T, Ito Y, Yamada T, Nozawa R. S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Curr Cancer Drug Targets. 2008;8(4):243–52.
Zha H, Sun H, Li X, Duan L, Li A, Gu Y, et al. S100A8 facilitates the migration of colorectal cancer cells through regulating macrophages in the inflammatory microenvironment. Oncol Rep. 2016;36(1):279–90. https://doi.org/10.3892/or.2016.4790.
Reckenbeil J, Kraus D, Probstmeier R, Allam JP, Novak N, Frentzen M, et al. Cellular distribution and gene expression pattern of Metastasin (S100A4), Calgranulin A (S100A8), and Calgranulin B (S100A9) in oral lesions as markers for molecular pathology. Cancer Investig. 2016;34(6):246–54. https://doi.org/10.1080/07357907.2016.1186172.
Acknowledgements
The authors thank the Department of Radiology, University of Bonn for professional and financial support of the project. The authors are greatly thank the study nurse Mrs. Olga Ramig (Department of Radiology, University of Bonn, Germany) for collecting the patient data and personal supervision of the patients over the entire time of the project.
Funding
The study funding has been performed by the Department of Radiology, University of Bonn, Germany. Kristina Yeghiazaryan has been awarded a research fellowship with the European Association for Predictive, Preventive and Personalised Medicine (EPMA, Brussels, Belgium).
Author information
Authors and Affiliations
Contributions
O. Golubnitschaja created the concept of the project, made the data interpretation and drafted the article. J. Polivka Jr. carried out the statistical evaluation and graphical presentation of the data collected contributing significantly to the final version of the article. K. Yeghiazaryan carried out the molecular biological investigations. L. Berliner contributed to the data interpretation. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
All the patients were informed about the purposes of the study and consequently have signed their “consent of the patient”. All investigations conformed to the principles outlined in the Declaration of Helsinki and were performed with permission from the responsible Ethical Committee of the Medical Faculty, Rheinische Friedrich-Wilhelms-University of Bonn. Corresponding reference number is 283/10.
Rights and permissions
About this article
Cite this article
Golubnitschaja, O., Polivka, J., Yeghiazaryan, K. et al. Liquid biopsy and multiparametric analysis in management of liver malignancies: new concepts of the patient stratification and prognostic approach. EPMA Journal 9, 271–285 (2018). https://doi.org/10.1007/s13167-018-0146-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-018-0146-6