Abstract
Extracts of five organic and one conventional honey sources, available in Finnish markets, were tested for antimicrobial activity and inhibitory concentrations against Escherichia coli, Salmonella Typhi, Pseudomonas aeruginosa, Klebsiella pneumoniae, Bacillus cereus, and Staphylococcus epidermidis, obtained from human specimens. Five (honeys A, B, D, E, F) of the six studied honeys were organic. All the studied honeys had inhibitory activity (zone of inhibition (ZI) > 9.4 ± 0.5 mm) compared to control artificial honey (ZI < 8 mm) against all the studied pathogens. Four organic honeys (B, D, E, F) showed inhibitory activity with ZI > 13.3 mm against all the studied bacteria with minimal inhibitory concentration (MIC) values of 12.5–50%. Against E. coli, the organic honeys E and F had activity index (AI) of 0.64 and 0.73, respectively, compared to the antibiotic AI of 1.0. Against S. Typhi, the organic honeys D and F had AI of 0.59 and 0.64, respectively. Against P. aeruginosa, the organic honeys D, E, and F had the highest AI of 0.71–0.80, and against S. epidermidis the honeys B, D, E, and F possessed relatively high AI of 0.60, 0.67, 0.73, and 0.78, respectively. Against K. pneumoniae and B. cereus, the detected AI of the organic honeys B, D, E, and F varied between AI of 0.48 and 0.58. The organic honey A and conventionally produced honey C possessed only minor activity with MIC values of 80%. Here, we show that commercially available culinary organic honeys possess remarkable antimicrobial activity against several important human bacterial pathogens.
Similar content being viewed by others
Background
Antibiotic-resistant bacteria and their resistance genes emerge and spread globally among people, food, animals, plants, and the environment (soil, water, and air) (Berendonk et al. 2015). Erratic success in treating infectious diseases results in important societal and economic costs to human health and well-being (WHO 2020). Excessive and improper use of antibiotics in farm animals amplify and accelerate this development (Manyi-Loh et al. 2018). Introducing a novel antimicrobial requires lengthy efficacy and safety studies before introduction entry into the market. Increasing worldwide emergence of multidrug-resistant pathogens emphasizes the need to develop alternative or complementary treatment strategies, effective substances, formulas, or active ingredients.
Honey is a natural sweet substance consisting of hundreds of compounds (Maddocks and Jenkins 2013; Nolan et al. 2019). Honey has been used both as food and as a traditional medicine for centuries (Zumla and Lulat 1989). Comprehensive reviews and several studies have been published on honey varieties with promising antibacterial and medicinal properties (Maddocks and Jenkins 2013, Zainol et al. 2013, Huttunen et al. 2013, Salonen et al. 2017, Mandal and Mandal 2011, Combarros-Fuertes et al. 2020), and especially in wound healing (Al-Waili et al. 2011). Recently, a meta-analysis confirmed the activity of honey against viral respiratory infections (Abuelgasim et al. 2020), and immunomodulatory effects of different sources of honeys have also been demonstrated (Ota et al. 2019). Honeys have not been reported to have toxic or other harmful side effects (Zainol et al. 2013). There are several reports showing activity of honey against antibiotic-resistant bacteria (Kwakman et al. 2008, Cooper et al. 2010, Maddocks and Jenkins 2013, Huttunen et al. 2013, Shah Pratibha and Williamson Manita 2015, Natarajan et al. 2001).
Difference in antimicrobial potency among different sources of honey can be more than 100-fold, depending on their geographical, seasonal, and botanical origin, as well as harvesting, processing, and storage conditions (Molan and Cooper 2000). The antibacterial nature of honey is dependent on various factors working either individually or synergistically. High osmolarity (Kwakman and Zaat 2012), low pH (Karabagias et al. 2014), and hydrogen peroxide (White et al. 1963, Kwakman and Zaat 2012, Brudzynski 2020) are the main antimicrobial factors (Molan 1992, 2001, reviewed by Combarros-Fuertes et al. 2020). Phenolic compounds may contribute to antimicrobial activity (Estevinho et al. 2008; Agbaje et al. 2006). Additional antimicrobial mechanisms have been detected from Revamil® and Manuka honeys (Kwakman et al. 2011). The main active component in Manuka honey is methylglyoxal with concentrations from 38.4 to 761 mgkg− 1 (Mavric et al. 2008). An antimicrobial peptide, bee defensin-1, has been identified from Revamil® honey (Kwakman et al. 2010).
Honey is a food commodity frequently involved in food fraud cases (Aries et al. 2016). Adulteration by sweeteners is one of the most common authenticity issues. The simplest way to adulterate honey involves the addition of sugar (syrups) directly to honey. In 2015, EU countries together with Norway and Switzerland assessed both the prevalence of sugar-adulterated honeys on the market, and honeys mislabeled with regard to botanical source or geographical origin. Of the tested honey samples collected at all stages of the supply chain, 14% contained added sugar. The practice of adding sugars to honey is occurring both within the EU and in the third world (Aries et al. 2016). A study with Australian honeys has shown that about 27% of the commercially available honeys tested were of “questionable authenticity” (Zhou et al. 2018). Consumers who buy honey from the supermarkets are not able to distinguish possible fraudulence present in food markets. Even chemical analyses are complicated for detection of fraudulent honey.
Research on antimicrobial activity of organic honeys is a novel approach and only a few studies exist regarding bioactivity or quality. Quality of 75 organic Erica species sourced from the Trás-Os-Montes region (Portugal) has been evaluated along with measurement of bioactivity and content of phenols and flavonoids (Estevinho et al. 2012) and found to be relatively high and comparable to previous studies with non-organic Erica sp. honeys (Feás et al. 2013). In our earlier studies, we have shown that Finnish organic honeys have antibacterial activity against the food pathogen Clostridium perfringens (Oinaala et al. 2015). Clinically used SurgihoneyRO™, which is engineered from organic honey, effectively prevents biofilm formation by clinically important wound pathogens in vitro (Halstead et al. 2017). Previously unknown mechanisms and factors have been detected from organic honeys. Honey extracts from organic floral sources had favorable effects on pesticide‐induced DNA damage response (Alleva et al. 2016).
The present study was made considering consumer perspectives and bioactivity among randomly selected organic honeys available in supermarkets. Finnish and foreign culinary commercial organic honeys from supermarkets in Helsinki were investigated. They were tested for their antibacterial activity against six important bacterial pathogens isolated from different clinical specimens in Kenia. The studied medically important bacteria Escherichia coli, Salmonella Typhi, Pseudomonas aeruginosa, Klebsiella pneumoniae, Bacillus cereus, and Staphylococcus epidermidis represent various disease conditions. E. coli is a versatile bacterial species with the ability to cause intestinal or systemic diseases in humans and in animals (Leimbach et al. 2013). E. coli is associated with urinary tract infections, diarrhea, septicemia, wound, and other infections, such as neonatal meningitis. E. coli causes bacterial infections in humans and is prominently associated with diarrhea in pets and farm animals. The therapeutic treatment is compromised by the emergence of antimicrobial resistance (Allocati et al. 2013). Human infections with typhoidal Salmonella serovars (Salmonella Typhi and Salmonella Paratyphi A) cause typhoid fever, the treatment of which is complicated by increasing drug resistance (Johnson et al. 2018). Typhoidal serovars cause a systemic infection (Raffatellu et al. 2008). P. aeruginosa causes infections and diseases in both plants and animals, including several human diseases, e.g., wound infections, diabetic foot ulcers, urinary infections, and many hospital-acquired infections especially in immune-compromised patients. P. aeruginosa is an opportunistic pathogen and the occurrence of antimicrobial resistance makes it difficult to treat and eradicate (Azam and Khan 2019). K. pneumoniae, known as a major threat to public health, is the most common factor of hospital- and community-acquired infections. Clinical specimens can be isolated from bronchial, urea, blood, catheter, rectal, bile, tracheal, and wound cultures (Shah Pratibha and Williamson Manita 2015). B. cereus has been associated with food poisoning. The bacterium causes two types of gastrointestinal disease, the diarrheal and the emetic syndromes, which are caused by very different types of toxins (Stenfors Arnesen et al. 2008). S. epidermidis is an important commensal organism of the human skin and mucous membranes. S. epidermidis can cause opportunistic infections including biofilm-associated infections on indwelling medical devices and nosocomial sepsis (Nguyen et al. 2017). S. epidermidis can be found in food products, e.g., artisanal cheeses from raw whole cow milk, being a threat for humans with more virulence factors and antibiotic resistance through mobile genetic elements (Chajęcka-Wierzchowska et al. 2019).
Great variabilities of honey samples regarding quality and bioactivity are available for customer use in the market. Organic honeys are produced using strict ecological and natural principles which are meant to enhance the good quality (Estevinho et al. 2012). Antimicrobial activity of the organic honeys is less studied. The aim of the present study was to collect commercial organic honey samples from Finnish supermarkets in order to investigate their antimicrobial activity against important human pathogens.
Materials and methods
Materials
Preparation of honey samples and antibiotics
Six honey samples were randomly purchased from supermarkets in Helsinki, Finland (Table 1), and were randomly coded from A to F. According to the labelling, five of the honey samples (A, B, D, E, and F) were organic and one (C) was non-organic. Two of the honeys originated from Finland (C and E) and one honey sample (B) was a mixture of organic honeys (unknown proportions) from Bulgaria and Romania. Honeys A, D, and F were produced outside of EU. Floral sources were indicated for honeys E and A. Honey A was produced from the nectar of agave flowers. The nectar of honey E had been collected from forest raspberry, bilberry, lingonberry, and willow herb. Honey C had been filtered free from pollen and honey E had been kneaded (mechanically stirred slowly in order to generate small honey crystals). The other studied honeys were in their raw form. Four additional honey samples were collected from Nandi County villages of Kenya for antimicrobial and methylglyoxal assessment.
The honey samples were stored in the dark at + 4 °C. Each honey sample was diluted in sterile deionized water to achieve concentrations of 80, 50, 25, 12.5, and 6.25% (w/v). The samples were used immediately after dilution. The control antibiotic used for the study was gentamycin (Unice Pharma, China) (10 µg) against K. pneumoniae, B. cereus, and S. epidermidis and ciprofloxacin (Cipla Ltd, India) (10 µg) against E. coli, S. Typhi, and P. aeruginosa. Sterile deionized water was used as a negative control. An artificial honey control was also prepared to test the osmolarity of the honey sugars against the organisms. The control contained 40 g of fructose, 30 g of glucose, 8 g of maltose, and 2 g of sucrose dissolved in 100 ml of distilled water and diluted to obtain concentrations of 80,50, 25, 12.5, and 6.25% (w/v).
Preparation of bacterial inoculum
The studied six clinical isolates of pathogenic bacteria originated from different clinical specimens obtained from the Baraton University Hospital, Baraton, Kenya. The bacterial strains were identified by the Department of Medical Laboratory Sciences, University of Eastern Africa, Baraton, Kenya, with standard laboratory tests (Chauhan and Jindal 2020; Shoaib et al. 2020) applying ISO TC/212 (Clinical laboratory testing and in vitro diagnostic test systems) regulation on laboratory standards. The bacterial organisms were Escherichia coli and Salmonella Typhi from stool, Pseudomonas aeruginosa from an infected wound, Bacillus cereus from spoiled rice, Staphylococcus epidermidis from a wound, and Klebsiella pneumoniae isolated from urine. E. coli, S. Typhi, B. cereus, and P. aeruginosa were cultured on Mueller Hinton agar (HiMedia, India) and S. epidermidis and K. pneumoniae on blood agar (HiMedia, India). Each organism was re-suspended in Mueller Hinton broth (HiMedia, India) and subjected to 37 °C overnight. The bacterial inoculum for each species was standardized to 1.0 × 108 cfu/ml using a 0.5 McFarland’s Standard and a UV–Vis spectrophotometer.
Antimicrobial activity
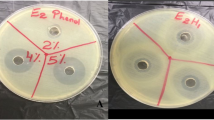
Antimicrobial activity of all the honey samples against each of the bacterial organisms was examined in Kenya using the agar well diffusion method (Rajeswari et al. 2010, Sherlock et al. 2010, Oinaala et al. 2015, Stagos et al. 2018, Mgbeahuruike et al. 2018).
The organisms were separately plated on Mueller Hinton agar after an overnight culture in Mueller Hinton broth. A sterile cork bore of 6-mm diameter was used to produce the wells on the agar plates. Each honey sample was placed in wells to obtain a replicate of nine readings per sample. An artificial honey control, as well as positive and negative controls, was examined for each organism tested. Antimicrobial activity of the six honeys was measured as zone of inhibition (ZI) in millimeters with the aid of a ruler. Approximate minimum inhibitory concentrations (MIC) were determined by the agar well diffusion method (Oinaala et al. 2015). Five different concentrations, 80, 50, 25, 12.5, and 6.25% (w/v), of each honey were used to determine the lowest honey concentration that gives ZI > 6 mm. The activity index (AI) is expressed as follows: inhibition zone of the honey/ inhibition zone of the antibiotic control.
Quantification of methylglyoxal
For the four honey samples from Nandi County villages of Kenya, methylglyoxal (MGO) analysis was carried out in Finland and determined as quinoxaline derivative as described before for Finnish organic honeys (Oinaala et al. 2015). The honeys were treated with o-phenylenediamine (OPD), which reacts with MGO and forms quinoxaline derivative, 2-methylquinoxaline (MQ). Briefly, honeys (3.0 g) were dissolved in 20 ml of 0.5 M phosphate buffer (pH 6.5) containing 1% (w/v) of OPD and 0.3 mg of p-cymene (PC) (internal standard). Reactions were performed in the dark at room temperature for 12 h. Samples were extracted three times with 4 ml of dichloromethane, dried over Na2SO4, and evaporated to 0.5 ml. The analyses of derivatized samples were performed with a GC/MS system (Agilent Technologies) consisting of a gas chromatograph (series 7890 A), an autosampler (series 7693), and a mass selective detector (series 5975 C). The column was HP5-MS (5% phenylmethylsiloxane silica capillary column, 30 m × 250 µm × 0, 25 µm). The concentrations were obtained based on internal standard calibration. Calibration was performed with five standards each containing 0.3 mg of PC. Standards were prepared from MGO and they were treated and analyzed like samples. The GC oven was programmed to increase the temperature from the initial temperature of 40 to 208 °C at the rate of 4 °C/min. The mass spectra and the retention time (20.7 min) were confirmed with a pure MGO compound. MGO, OPD, MQ, and PC were obtained from Sigma Aldrich, Finland. The other reagents used were from VWR International, Finland. Water used for sample and solution preparations was obtained from Millipore Synergy water purification system.
Results
Antimicrobial activity
The detected antimicrobial activity was dose dependent regardless of the honey sample. All the studied honeys possessed antimicrobial activity against the studied bacterial pathogens (ZI > 9.4 ± 0.5 mm, AI > 0.34) compared to the control artificial honey (ZI < 8 mm, AI < 0.29) (Table 2). The most active honeys were the honeys F and E, then the honeys D and B in that order (Tables 2 and 3) with the MIC values of 12.5–50% (Table 4). The lowest MICs were detected for the organic honey F, produced in South and Middle America and for the organic honey E of Finnish origin (MICs for F and E 12.5–25%), followed by the organic honey D packed in Denmark and the organic honey B from Bulgaria/Romania (MICs for D and B 12.5–50%) (Table 4). Compared to the antibiotic activity index (AI = 1.0), the organic honeys D, E, and F had high inhibitory activity against P. aeruginosa (ZI > 18.3 ± 0.5 mm, AI 0.71–0.80) and against S. epidermidis (ZI > 18.7 ± 0.9 mm, AI 0.67–0.78) (Table 2, Table 3). Against E. coli, the organic honey E (ZI 17.9 ± 0.3, AI 0.64) and the organic honey F (ZI 20.7 ± 0.5, AI 0.73) had remarkable activity (Table 2, Table 3). The organic honey F had higher activity against S. Typhi (ZI 15.7 ± 0.5, AI 0.64) compared to lower activity of the organic honeys A, B, D, and E (ZI 12.7 ± 0.5 –14.0 ± 0.0 mm, AI 0.52–0.59) (Table 2, Table 3). The organic honeys A, B, D, E and F induced some growth inhibition against B. cereus (ZI 11.9 ± 0.3 – 15.0 ± 0.5 mm, AI 0.46–0.58). The organic honey A showed low inhibitory activity against P. aeruginosa (ZI 12.8 ± 0.4, AI 0.50) and against E. coli (ZI 12.4 ± 0.5 mm, AI 0.44) (Tables 2 and 3). The non-organic honey C had low inhibitory activity against the studied bacteria (ZI 9.4 ± 0.5 – 12.8 ± 1.0 mm, AI 0.34–0.46) (Tables 2 and 3). The organic honey A and the non-organic honey C showed activity against all the studied bacteria only at a concentration of 80% and higher (Table 4). The MIC is the lowest concentration of the honey that caused ZI > 6 mm.
The four honey samples collected from Kenyan County villages revealed no antimicrobial activity against any of the studied bacteria (Table 5).
MGO contents
MGO concentrations in the four honeys collected from Kenyan villages were between 7 and 29 mg/kg in each of the four honey samples (Table 5).
Discussion and conclusions
Numerous studies carried out with conventionally produced honeys from different countries and especially with New Zealand Manuka honey have proved that honey possesses significant antibacterial activity against several bacterial pathogens, also against E. coli, S. Typhi, P. aeruginosa, K. pneumoniae, B. cereus, and S. epidermidis (Mandal and Mandal 2011; Feás et al. 2013; Salonen et al. 2017; Matzen et al. 2018; Combarros-Fuertes et al. 2019). In the present study, we show that all the studied commercial honeys had antibacterial activity against human pathogenic organisms E. coli, S. Typhi, P. aeruginosa, K. pneumoniae, B. cereus, and S. epidermidis responsible for various human diseases. The studied pathogens were susceptible to four of the five organic honeys (B, D, E, and F) at 12.5–50% concentrations. The most active honeys were organic honey F, produced in South and Middle America, and organic honey E of Finnish origin, followed by organic honey D packed in Denmark and organic honey B from Bulgaria/Romania. Organic honey A and non-organic honey C were effective at 80–100% concentrations and possessed only minor antimicrobial activity. There were no reported heat treatments. Honey C was described being filtered to remove pollen and the floral source was not indicated. The floral source for honey A was agave, which has earlier reported negative regarding antibacterial activity (Matzen et al. 2018). The reason for the weak activity of the organic agave honey A and conventionally produced honey C against the studied bacterial pathogens remains open. The small number of honey samples does not allow comparison of the antibacterial activity between organic and non-organic honeys.
Honey composition is variable and depends on the floral source and on external factors, such as seasonal and environmental, as well as processing, manipulation, packaging, and storage conditions (Da Silva et al. 2016; Combarros-Fuertes et al. 2020). Heated or processed honeys can be active as shown by Huttunen et al. (2013), Lee and Lee (2016), and Ayub et al. (2020). Dose-dependent antimicrobial effect was observed against E. coli, S. Typhi, and K. pneumoniae with increased concentrations (6.25–50% v/v) of autoclaved Marhaba branded honey and unbranded local Pakistanian honey from Ziziphus mauritiana plant (Ayub et al. 2020). Different solvent fractions of Korean domestic honey had antimicrobial activity against B. cereus strains. Honey, as also shown in the present study, could be a good source for the natural antimicrobials used in the food industry and other related industries against food poisoning pathogens like B. cereus. In the present study, E. coli and S. Typhi were isolated from stool, B. cereus from spoiled rice, and K. pneumoniae from urine. Antibacterial activity of the studied commercial organic honeys against these pathogens suggests the protective effect of organic culinary honeys against diarrhea (Oinaala et al. 2015, Lee and Lee 2016) and against urinary tract infections as described before for non-organic honeys (Bouacha et al. 2018). In the present study, antibacterial activity was detected from organic honeys against S. epidermidis and P. aeruginosa obtained from wound. Organic honeys thus show further potential for medical products against wound infections like medical SurgihoneyRO™ in clinical use (Halstead et al. 2017).
In the present study, the four honeys collected from different villages in Kenyan Nandi County did not possess antimicrobial activity against the studied pathogens. The analyzed MGO concentrations of the honeys varied from 5 to 29 mgkg−1. The relatively low MGO concentrations of the local honeys from Nandi County can be of natural origin or due to the dilution of the honeys with sugar or other substances to achieve maximal profit from the sales (Aries et al. 2016). In our previous studies, MGO amounts of 22–27 mgkg−1 were found from Finnish antimicrobial organic honeys with no correlation to the detected antimicrobial activity (Oinaala et al. 2015). In Nordic forest honeys, high levels of MGO (up to 166 mg kg−1) have been found, but the mechanisms of the antibacterial activities could not be explained by MGO content only or by any other individual factor associated with different honeys (Salonen et al. 2017). Our present results support the previous studies (Oinaala et al. 2015) that low concentrations of MGO have no impact on antimicrobial activity and if present is caused by other often unknown factors. Mokaya et al. (2020) analyzed different types of Kenyan honeys. The studied honeys inhibited growth of E. coli and showed substantial amount of nonperoxide antimicrobial activity and suggested that Kenyan honeys have great therapeutic potential. They reported that geographical origin of honey had an influence on its bioactive contents, which may be the reason for the inactivity detected from honeys from Nandi County in the present study.
Efficacy of conventionally produced honey against pathogenic bacteria has been scientifically demonstrated (reviewed by Mandal and Mandal 2011). Honey is composed of hundreds of compounds, any one of which may act at several sites, additively or synergistically. Apparently, resistance to honey properties is therefore difficult to develop (Combarros-Fuertes et al. 2019). When bioactive honey compounds are used separately, at the concentrations detected in honey, they may not have an effect on bacteria (Combarros-Fuertes et al. 2019).
Honey also acts synergistically with several antibiotics, reducing the doses required to inhibit bacterial growth or reverting the antibiotic-resistance previously acquired (Jenkins and Cooper 2012, Campeau and Patel 2014, Hayes et al. 2018). In order to propose honey as a good antibacterial agent in the food and for the food processing, further investigations to determine the tolerance of honeys to low pH and high temperature will be necessary (Ayub et al. 2020). Controlled, large-scale clinical trials will be needed to confirm honeys’ efficacy in vivo as regards antimicrobial and possible immunomodulatory impact of honeys. Application of honey for clinical purposes needs standardization of both antimicrobial activity and bioactive composition against bacterial, viral, and fungal pathogens (Al-Waili et al. 2011, Kwakman and Zaat 2012, Combarros-Fuertes et al. 2019).
In order to ensure safety of honey especially for medical uses, it must be free of any form of contamination, such as herbicides, pesticides, heavy metals, and spores. To meet these criteria, honey must be collected in organic regions, as well as following strict quality, processing, and storage standards and regulations (Nair et al. 2020), where organic agriculture opens a huge possibility. Consumers use honey mostly as sweetener and for culinary purposes, and have also raised interest regarding health impacts of honey, especially its activity against viral flu (Watanabe et al. 2014; Abuelgasim et al. 2020) and against respiratory infections (Huttunen et al. 2013). Commercial honeys for culinary purposes may be raw or processed. Processed, e.g., heated, honeys may be inactive (Matzen et al. 2018) or may preserve even after heating (Huttunen et al. 2013; Ayub et al. 2020), an option for organic food processing or medical purposes without gamma radiation.
The emergence and rapid spread of viruses and antibiotic resistant bacteria are of concern for human health and human-connected animals, farms, food, water, and natural ecosystems worldwide. Here, we detected significant antimicrobial activity against six important human pathogens from four of five studied organic culinary honeys available in supermarkets for consumer use. This activity is comparable to the activity previously shown for conventionally produced honeys in numerous reports (reviewed by Mandal and Mandal 2011; Huttunen et al. 2013; Salonen et al. 2017; Matzen et al. 2018; Combarros-Fuertes et al. 2019). Our present study shows that commercially available culinary organic honeys from different sources and countries possess remarkable antimicrobial activity against various important human bacterial pathogens. Organic honeys carry potential for consumer use with anti-infective preventive impacts. They also could be applied in food industry for the development of novel antimicrobial food products.
Data availability
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author, CTK, and the principal author JKO, upon reasonable request.
Code availability
Not applicable.
References
Abuelgasim H, Albury C, Lee J (2020) Effectiveness of honey for symptomatic relief in upper respiratory tract infections: a systematic review and meta-analysis. BMJ Evid Based Med 18:bmjebm-2020–111336. https://doi.org/10.1136/bmjebm-2020-111336
Agbaje EO, Ogunsanya T, Aiwerioba OIR (2006) Conventional use of honey as antibacterial agent. Ann Afr Med 5:79–81
Alleva R, Manzella N, Gaetani S, Ciarapica V, Bracci M, Caboni MF, Pasini F, Monaco F, Amati M, Borghi B, Tomasetti M (2016) Organic honey supplementation reverses pesticide-induced genotoxicity by modulating DNA damage response. Mol Nutr Food Res 10:2243–2255. https://doi.org/10.1002/mnfr.201600005
Allocati N, Masulli M, Alexeyev MF, Di Ilio C (2013) Escherichia coli in Europe: an overview. Int J Environ Res Public Health 10:6235–6245. https://doi.org/10.3390/ijerph10126235
Al-Waili NS, Salom K, Butler G, Al Ghamdi AA (2011) Honey and microbial infections: a review supporting the use of honey for microbial control. J Med Food 14:1079–1096. https://doi.org/10.1089/jmf.2010.0161
Aries E, Bourton J, Carrasco L, De Rudder O, Maquet A (2016) Scientific support to the implementation of a coordinated control plan with a view to establishing the prevalence of fraudulent practices in the marketing of honey. Results of honey authenticity testing by liquid chromatography-isotope ratio mass spectrometry. Administrative Arrangement N° SANTE/2015/E3/JRC/SI2.706828 JRC Technical Reports, European Commission. https://ec.europa.eu/food/system/files/2017-03/oc_control-progs_honey_jrc-tech-report_2016.pdf. Accessed 21 Jan 2021
Ayub R, Umer M, Maan AA, Rasool B, Khan MKI, Younis T, Abbas S, Sajjad M, Kaleem I, Imran M, Ullah A, Afzal MS, Shah ZH, Ahmed S, Aslam F, Chaudhary N, Afzal MI (2020) Antibiotics, acid and heat tolerance of adapted Escherichia coli, Salmonella Typhi and Klebsiella pneumoniae. Foods 9:311. https://doi.org/10.3390/foods9030311
Azam MW, Khan AU (2019) Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov Today 24:350–359. https://doi.org/10.1016/j.drudis.2018.07.003
Berendonk TU, Manaia CM, Merlin C, Fatta-Kassinos D, Cytryn E, Walsh F, Bürgmann H, Sørum H, Norström M, Pons MN, Kreuzinger N, Huovinen P, Stefani S, Schwartz T, Kisand V, Baquero F, Martinez JL (2015) Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol 13:310–317. https://doi.org/10.1038/nrmicro3439
Bouacha M, Ayed H, Grara N (2018) Honey bee as alternative medicine to treat eleven multidrug-resistant bacteria causing urinary tract infection during pregnancy. Sci Pharm. https://doi.org/10.3390/scipharm86020014
Brudzynski K (2020) A current perspective on hydrogen peroxide production in honey. A Review Food Chem 332:127229. https://doi.org/10.1016/j.foodchem.2020.127229
Campeau MEM, Patel R (2014) Antibiofilm activity of Manuka honey in combination with antibiotics. Int J Bacteriol. https://doi.org/10.1155/2014/795281
Chajęcka-Wierzchowska W, Zadernowska A, Gajewska J (2019) S. epidermidis strains from artisanal cheese made from unpasteurized milk in Poland - genetic characterization of antimicrobial resistance and virulence determinants. Int J Food Microbiol 2:55–59. https://doi.org/10.1016/j.ijfoodmicro.2019.02.004
Chauhan A, Jindal T (2020) Biochemical and molecular methods for bacterial identification. In: Microbiological methods for environment, food and pharmaceutical analysis. Springer, Cham, pp 425–468. https://doi.org/10.1007/978-3-030-52024-3_10
Combarros-Fuertes P, Estevinho LM, Dias LG, Castro JM, Tomás-Barberán FA, Tornadijo ME, Fresno-Baro JM (2019) Bioactive components and antioxidant and antibacterial activities of different varieties of honey: a screening prior to clinical application. J Agric Food Chem 67:688–698. https://doi.org/10.1021/acs.jafc.8b05436
Combarros-Fuertes P, Fresno JM, Estevinho MM, Sousa-Pimenta M, Tornadijo ME, Estevinho LM (2020) Honey: another alternative in the fight against antibiotic-resistant bacteria? Antibiotics (basel). https://doi.org/10.3390/antibiotics9110774
Cooper RA, Jenkins L, Henriques AF, Duggan RS, Burton NF (2010) Absence of bacterial resistance to medical-grade Manuka honey. Eur J Clin Microbiol Infect Dis 29:1237–1241
Da Silva PM, Gauche C, Gonzaga LV, Costa ACO, Fett R (2016) Honey: chemical composition, stability and authenticity. Food Chem 196:309–323. https://doi.org/10.1016/j.foodchem.2015.09.051
Estevinho LM, Feás X, Seijas JA, Pilar Vázquez-Tato M (2012) Organic honey from Trás-Os-Montes region (Portugal): chemical, palynological, microbiological and bioactive compounds characterization. Food Chem Toxicol 50:258–264. https://doi.org/10.1016/j.fct.2011.10.034
Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E (2008) Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol 46:3774–4377
Feás X, Iglesias A, Rodrigues S, Estevinho LM (2013) Effect of Erica sp. honey against microorganisms of clinical importance: study of the factors underlying this biological activity. Molecules (Basel, Switzerland) 18:4233–4246. https://doi.org/10.3390/molecules18044233
Halstead FD, Webber MA, Oppenheim BA (2017) Use of an engineered honey to eradicate preformed biofilms of important wound pathogens: an in vitro study. J Wound Care 26:442–450. https://doi.org/10.12968/jowc.2017.26.8.442
Hayes G, Wright N, Gardner SL, Telzrow CL, Wommack AJ, Vigueira PA (2018) Manuka honey and methylglyoxal increase the sensitivity of Staphylococcus aureus to linezolid. Lett Appl Microbiol 66:491–495. https://doi.org/10.1111/lam.12880
Huttunen S, Riihinen K, Kauhanen J, Tikkanen-Kaukanen C (2013) Antimicrobial activity of different Finnish monofloral honeys against human pathogenic bacteria. APMIS 121:827–834. https://doi.org/10.1111/apm.12039
Jenkins R, Cooper R (2012) Improving antibiotic activity against wound pathogens with Manuka honey in vitro. PLoS ONE. https://doi.org/10.1371/journal.pone.00456007
Johnson R, Mylona E, Frankel G (2018) Typhoidal Salmonella: distinctive virulence factors and pathogenesis. Cell Microbiol. https://doi.org/10.1111/cmi.12939
Karabagias IK, Vavoura MV, Nikolaou C, Badeka AV, Kontakos S, Kontominas MG (2014) Floral authentication of Greek unifloral honeys based on the combination of phenolic compounds, physicochemical parameters and chemometrics. Food Res Int 62:753–760. https://doi.org/10.1016/j.foodres.2014.04.015
Kwakman PH, Van den Akker JP, Güçlü A, Aslami H, Binnekade JM, de Boer L, Boszhard L, Paulus F, Middelhoek P, te Velde AA, Vandenbroucke-Grauls CM, Schultz MJ, Zaat SA (2008) Medical-grade honey kills antibiotic-resistant bacteria in vitro and eradicates skin colonization. Clin Infect Dis 46:1677–1682. https://doi.org/10.1086/587892
Kwakman PH, Zaat SA (2012) Antibacterial components of honey. IUBMB Life 64:48–55. https://doi.org/10.1002/iub.578
Kwakman PHS, te Velde AA, de Boer L, Speijer D, VandenBroucke-Grauls CMJE, Zaat AJ (2010) How honey kills bacteria. FASEB J 24:2567–2582
Kwakman PHS, te Velde AA, de Boer L, VandenBroucke-Grauls CMJE, Zaat SAJ (2011) Two major medicinal honeys have different mechanisms of bactericidal activity. PLoS ONE 6(3):e17709. https://doi.org/10.1371/journal.pone.0017709
Lee SK, Lee H (2016) Antimicrobial activity of solvent fractions and bacterial isolates of Korean domestic honey from different floral sources. Food Sci Biotechnol 25:1507–1512. https://doi.org/10.1007/s10068-016-0234-0
Leimbach A, Hacker J, Dobrindt U (2013) E. coli as an all-rounder: the thin line between commensalism and pathogenicity. Curr Top Microbiol Immunol 358:3–32. https://doi.org/10.1007/82_2012_303
Maddocks SE, Jenkins RE (2013) Honey: a sweet solution to the growing problem of antimicrobial resistance? Future Microbiol 8:1419–1429. https://doi.org/10.2217/fmb.13.105
Mandal MD, Mandal S (2011) Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed 1:154–160. https://doi.org/10.1016/S2221-1691(11)60016-6
Manyi-Loh CE, Mamphweli S, Meyer E, Okoh A (2018) Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 23:795. https://doi.org/10.3390/molecules23040795
Matzen RD, ZinckLeth-Espensen J, Jansson T, Nielsen DS, Lund MN, Matzen S (2018) The antibacterial effect in vitro of honey derived from various Danish flora. Dermatol Res Pract. https://doi.org/10.1155/2018/7021713
Mavric E, Wittmann S, Barth G, Henle T (2008) Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol Nutr Food Res 52:483–489
Mgbeahuruike EE, Fyhrquist P, Vuorela H, Julkunen-Tiitto R, Holm Y (2018) Alkaloid-rich crude extracts, fractions and piperamide alkaloids of Piper guineense possess promising antibacterial effects. Antibiotics (basel) 7:98. https://doi.org/10.3390/antibiotics7040098
Mokaya HO, Bargul JL, Irungu JW, Lattorff HMG (2020) Bioactive constituents, in vitro radical scavenging and antibacterial activities of selected Apis mellifera honey from Kenya. Int J Food Sci Technol 55:1246–1254. https://doi.org/10.1111/ijfs.14403
Molan P (1992) The antibacterial activity of honey 1. The nature of the antibacterial activity. Bee World 73:5–28
Molan P (2001) Why honey is effective as a medicine. Bee World 82:22–40
Molan PC, Cooper RA (2000) Honey and sugar as a dressing for wounds and ulcers. Trop Doct 30:249–250
Nair HKR, Tatavilis N, Pospíšilová I, Kučerová J, Cremers NAJ (2020) Medical-grade honey kills antibiotic-resistant bacteria and prevents amputation in diabetics with infected ulcers: a prospective case series. Antibiotics. https://doi.org/10.3390/antibiotics9090529
Natarajan S, Williamson D, Grey J, Harding KG, Cooper RA (2001) Healing of an MRSA-colonized, hydroxyurea-induced leg ulcer with honey. J Dermatol Treat 12:33–36. https://doi.org/10.1080/095466301750163563
Nguyen TH, Park MD, Otto M (2017) Host response to Staphylococcus epidermidis colonization and infections. Front Cell Infect Microbiol 21:90. https://doi.org/10.3389/fcimb.2017.00090
Nolan VC, Harrison J, Cox JAG (2019) Dissecting the antimicrobial composition of honey. Antibiotics. https://doi.org/10.3390/antibiotics8040251
Oinaala D, Lehesvaara M, Lyhs U, Tikkanen-Kaukanen C (2015) Antibacterial activity of organic Finnish honey against pathogenic bacteria. Org Agr 5:153–159. https://doi.org/10.1007/s13165-015-0103-9
Ota M, Ishiuchi K, Xu X, Minami M, Nagachi Y, Yagi-Utsumi M, Tabuchi Y et al (2019) The immunostimulatory effects and chemical characteristics of heated honey. J Ethnopharmacol 228:11–17. https://doi.org/10.1016/j.jep.2018.09.019
Raffatellu M, Wilson RP, Winter SE, Bäumler AJ (2008) Clinical pathogenesis of typhoid fever. J Infect Dev Ctries 2:260–266. https://doi.org/10.3855/jidc.219
Rajeswari T, Venugopal A, Viswanathan C, Kishmu L, Venil CK, Sasi kumar JM (2010) Antibacterial activity of honey against Staphylococcus aureus from infected wounds. Pharmacologyonline 1:537–541. https://pharmacologyonline.silae.it/files/newsletter/2010/vol1/59.Kumar.pdf. Accessed 21 Jan 2021
Salonen A, Virjamo V, Tammela P, Fauch L, Julkunen-Tiitto R (2017) Screening bioactivity and bioactive constituents of Nordic unifloral honeys. Food Chem 237:214–224
Shah Pratibha J, Williamson Manita T (2015) Antibacterial activity of honey against ESBL producing Klebsiella pneumoniae from burn wound infections. Int J Curr Pharm Res 7:32–36
Sherlock O, Dolan A, Athman R, Power A, Gethin G, Cowman S et al (2010) Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement Alternat Med 10:47. https://doi.org/10.1186/1472-6882-10-47
Shoaib M, Muzammil I, Hammad M, Bhutta ZA, Yaseen I (2020) Mini-review on commonly used biochemical tests for identification of bacteria. Int J Sci Res Publ 54(1). https://doi.org/10.47119/IJRP100541620201224
Stagos D, Soulitsiotis N, Tsadila C, Papaeconomou S, Arvanitis C, Ntontos A, Karkanta F, Adamou-Androulaki S, Petrotos K, Spandidos DA, Kouretas D, Mossialos D (2018) Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int J Mol Med 42:726–734. https://doi.org/10.3892/ijmm.2018.3656
Stenfors Arnesen LP, Fagerlund A, Granum PE (2008) From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 32:579–606. https://doi.org/10.1111/j.1574-6976.2008.00112.x
Watanabe K, Rahmasari R, Matsunaga A, Haruyama T, Kobayashi N (2014) Anti-influenza viral effects of honey in vitro: potent high activity of manuka honey. Arch Med Res 45:359–365. https://doi.org/10.1016/j.arcmed.2014.05.006.Erratum.In:ArchMedRes(2014)6:516
White JW, Subers MH, Schepartz AI (1963) The identification of inhibine, the antibacterial factor of honey, as hydrogen peroxide and its origin in honey glucose-oxidase system. Biochim Biophys Acta 73:57–70
World Health Organization (WHO) (2020) Antibiotic resistance Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance. Accessed 21 Jan 2021
Zainol MI, MohdYusof K, MohdYusof MY (2013) Antimicrobial activity of selected Malaysian honey. BMC Complement Altern Med. https://doi.org/10.1186/1472-6882-13-129
Zhou X, Taylor MP, Salouros H, Prasad S (2018) Authenticity and geographical origin of global honeys determined using carbon isotope ratios and trace elements. Sci Rep 8:14639. https://doi.org/10.1038/s41598-018-32764-w
Zumla A, Lulat A (1989) Honey – a remedy rediscovered. J R Soc Med 82:384–385
Acknowledgements
We thank the Baraton University Hospital for the bacterial strains, and the Department of Medical Laboratory Science, University of Eastern Africa, for the identification of the strains.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. We would like to thank the Department of Medical Laboratory Sciences, the University of Eastern Africa, Baraton, for allowing us to conduct the bioassay in its laboratory.
Author information
Authors and Affiliations
Contributions
Conceptualization: Jackie K Obey, Carina Tikkanen-Kaukanen. Methodology: Jackie K Obey, Moses M Ngeyiwa, Marjatta Lehesvaara. Formal analysis and investigation: Jackie K Obey, Moses M Ngeyiwa, Marjatta Lehesvaara. Writing–original draft preparation: Jackie K Obey, Carina Tikkanen-Kaukanen. Writing–critical review and editing: Atte von Wright, Jussi Kauhanen. Resources: Jackie K Obey. Supervision: Carina Tikkanen-Kaukanen, Atte von Wright, Jussi Kauhanen.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obey, J.K., Ngeiywa, M.M., Lehesvaara, M. et al. Antimicrobial activity of commercial organic honeys against clinical isolates of human pathogenic bacteria. Org. Agr. 12, 267–277 (2022). https://doi.org/10.1007/s13165-022-00389-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13165-022-00389-z