Abstract
The Atchafalaya River Basin (ARB) in southcentral Louisiana, USA, is a structurally and biotically diverse floodplain of Atchafalaya River (AR), which is the largest distributary of the Mississippi River. Annual floodplain inundation facilitates the exchange of nutrients and organic material between the AR and its floodplain, giving rise to the high productivity of the river-floodplain system. Production within the ARB is driven by periphytic algae, phytoplankton, and aquatic macrophytes, however, very little is known about periphytic algal assemblages in floodplain systems or how loss of annual flooding impacts these assemblages. In this study, we use artificial substrates to sample periphytic algae bi-weekly (January 2019 – September 2019) from ARB sites with active river connections and from a permanently-isolated floodplain system (Lake Verret). Our results showed that connection to the river caused spatiotemporal shifts in periphytic algal assemblages in the ARB. Overall, ARB sites had a higher density of algal cells compared with non-ARB sites, and for ARB sites with more active river connections, total algal density was greater nearer to river inputs, particularly for cyanobacteria and centric diatoms, with diatoms dominating periphyton assemblages year-round. In contrast, the river-isolated system was dominated largely by chlorophytes. In both isolated and connected systems, sites with heavy macrophyte cover showed increased densities of euglenoids, chrysophytes, and xanthophytes. Shifts in periphytic algal assemblages due to floodplain alterations, such as the disconnection of a floodplain from its river source, could impact higher trophic levels and should be considered in future wetland management decisions.
Antecedentes
La llanura aluvial del río Atchafalaya (ARB por sus siglas en inglés) en el centro-sur del estado de Louisiana, EEUU, es una llanura aluvial grande y diversa que rodea al río Atchafalaya (AR); el cuál es el distributario más grande del río Mississippi y recibe 30% del flujo total diario del Mississippi y el río Red juntos. La inundación anual fomenta el intercambio de nutrientes y material orgánico entre el AR y su llanura aluvial y se sospecha que da lugar a la gran productividad del sistema río-llanura aluvial. La producción primaria dentro del ARB se debe a las algas perifíticas, el plancton y los macrófitos acuáticos, sin embargo, no se sabe mucho sobre los grupos de algas perifíticas dentro de los sistemas de llanuras aluviales o de cómo las inundaciones anuales prolongadas afectan a estos grupos. En este estudio se utilizó un sustrato artificial para tomar muestras de las algas perifíticas de forma quincenal (enero -septiembre del 2019) de sitios dentro del ARB activamente conectados al río y de sitios en un lago de la llanura aluvial permanentemente aislado (lago Verret). Nuestros resultados demuestran que la inundación anual del río genera desplazamientos espacio-temporales de los grupos de algas perifíticas dentro del ARB. De forma general, los sitios dentro del ARB tuvieron mayor abundancia de algas comparados con los sitios fuera y, adicionalmente, la composición de las comunidades dentro de estos grupos también mostró diferencias importantes. Específicamente, en los sitios del ARB activamente conectados al río, la abundancia total de alga era mayor ente más cerca de las conexiones al rio estuvieran; particularmente, las cianobacterias, las algas diatomeas céntricas y las diatomeas fueron las principales componentes del perifiton durante todo el año. De forma contrastante, en el lago de la llanura aluvial aislados fueron predominantes las algas clorofíceas. En ambas regiones, los sitios con alta presencia de macrófitos demostraron mayor presencia de algas euglenoideas, criptofíceas y xantofíceas (sólo en el lago Verret). Los desplazamientos en los grupos de algas perifítica debidos a las perturbaciones a las llanuras aluviales, como la desconexión de la llanura aluvial de su rio fuente, podrían afectar los niveles tróficos superiores y afectar las decisiones en la gestión de los humedales en el futuro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
River-floodplain systems are ecologically and economically important and fulfill a number of ecosystem services, including primary and secondary production, as well as sediment and nutrient storage (Junk et al. 1989; Jardine et al. 2012, 2015; Pettit et al. 2017; Crook et al. 2019). Floodplains consist of a network of river-adjacent terrestrial habitats along with seasonally disconnected lakes and channels that become inundated during river flooding. Lateral connectivity between a river and its floodplain facilitates exchange of nutrients, organic material, fish, and other organisms that can move into newly available habitat (Junk et al. 1989; Pettit et al. 2017; Bayley et al. 2018). Macrophytes present in floodplain lakes and channels serve as refugia for macroinvertebrates, which are important prey items for riverine fishes. The abundance of food sources and reduced water velocity also make floodplain systems ideal for fish spawning and juvenile development. Thus, floodplains typically are highly productive and can support lucrative commercial fishing operations (Opperman et al. 2010).
In river floodplains, basal resources, like aquatic macrophytes and algae, are responsible for carbon fixation and incorporation of inorganic nutrients to upper trophic levels (Wetzel 1964; Campos-Silva et al. 2021; Cazzanelli et al. 2021). Historically, freshwater algal primary production was thought to be dominated mostly by phytoplankton (Reynolds 1994; Kalff 2002). However, the role of epiphytic algae has emerged as equally important (Wetzel 1983; Liboriussen and Jeppesen 2006; Adame et al. 2017). In river-floodplain systems, the role of epiphytic algae has not been widely studied, but the influx of inorganic nutrients (i.e., nitrate, nitrite, ammonium, phosphorus) onto the floodplain during the flood pulse (Bortolini et al. 2016) provides resources needed for growth and production of photosynthetic algae (Lewis et al. 2000; Ahearn et al. 2006; Sokal et al. 2010). Attached algae coexist with bacteria and organic material in complex matrices, creating a thin biofilm (i.e., periphyton) layer on submerged surfaces. These biofilms are the site of carbon and nutrient absorption and cycling (Wetzel 1964; Flemming 1993; Battin et al. 2016) and are sensitive to environmental changes (Mazumder et al. 2017). Anthropogenic changes that alter hydrologic regimes, such as dams and levees that lower the frequency and duration of flooding, can impact periphytic algal abundance, assemblage composition, and production (Agostinho et al. 2004, 2008).
Nearly all floodplains in the Northern Hemisphere have been anthropogenically altered (Lewis et al. 2000; Power et al. 2015), mostly for navigation or agricultural purposes or for flood control. Modifications to river-floodplain systems can have deleterious consequences for aquatic productivity and biodiversity. When floodplains become disconnected from their river sources through dam or levee construction, biological and chemical exchange between the river and floodplain is greatly reduced, threatening ecological integrity of the many processes in these systems that are tightly linked to flooding (Fernandes et al. 2009; Sokal et al. 2010; Algarte et al. 2016). Isolation from nearby water sources can severely limit organismal dispersal and can even lead to extirpation of sensitive species (Beisner et al. 2006; Shurin et al. 2009). The Yangtze River, for example, has been substantially altered to accommodate rising population needs, and many of its seasonally inundated lakes have been permanently severed from their river connections. These disconnected lakes show a substantial reduction in the diversity of riverine fishes, largely because of reduced access to habitat, complete loss of fluvial environments, and limited access to spawning grounds (Liu and Wang 2010). Jiang et al. (2020) recently studied fish populations in connected and disconnected lakes in the Yangtze River floodplain and found that fish populations in disconnected lakes had lower levels of taxonomic distinctiveness than populations inhabiting lakes with active river connections. In the Paraná River, Brazil, isolated floodplain lakes had greater environmental heterogeneity and higher levels of dissimilarity in macrophyte composition relative to seasonally connected lakes (Quirino et al. 2019). In addition, the diet of the invertivorous fish Moenkhausia bonita differed among isolated lakes, but not in connected lakes, indicating river connectivity was essential to food dispersal in these aquatic systems (Quirino et al. 2019).
River connectivity is important for algal communities as well. In floodplain lakes with active riverine connections, periphyton communities had a higher degree of species richness compared to isolated lakes (Agostinho et al. 2008). Similar results were found for species composition of free-floating algae (Lansac-Toha et al. 2016) as well as zooplankton, which feed on phytoplankton and have the potential to significantly influence assemblage dynamics (Li et al. 2019). In Brazil, phytoplankton richness and diversity were higher in lakes with active river links due to increased exchange of riverine algal species and transfer of nutrients (Bortolini et al. 2016).
The Atchafalaya River is the fifth largest river by discharge on the North American continent and is the main distributary of the Mississippi River (Ford and Nyman 2011; Piazza 2014). The Atchafalaya River Basin (ARB) supports a tremendous diversity of terrestrial, semi-aquatic, and aquatic species, thought to be fueled by river flooding events (Rutherford et al. 2001; Colon-Gaud et al. 2004; Troutman et al. 2007). Floods vary annually in degree and magnitude, but will typically inundate floodplain habitats, such as bayous, floodplain lakes, and excavated canals, for periods ranging from weeks to months. This pulse facilitates nutrient and organism exchange and drives the enormous production and biodiversity characteristic of this system, which supports numerous commercial fishing enterprises that generate approximately $17 million in fish and crayfish annually (NOAA 2018). Over the last several decades, the Atchafalaya River and its basin have undergone substantial hydrologic modification. Once over 8,000 km2, the ARB has been constricted to just half of its historic size (Sabo et al. 1999; Piazza 2014). Permanent lakes, bayous, and dredged channels on the Atchafalaya River floodplain support a diverse assemblage of native and exotic macrophytes (Walley 2007), which in turn provide substrate for highly productive periphyton assemblages, as well as the organisms that exploit this rich food source (e.g., Colon-Gaud et al. 2004; Fisher et al. 2012).
In this study, we explored how river flooding in this modified system impacted periphytic algal assemblages. We compared periphyton composition in ARB sites with active floodplain connections to a permanently-isolated floodplain system, Lake Verret (LV). We hypothesized that, relative to sites receiving no annual water inputs from a flood pulse, floodplain sites would: 1) have substantially greater periphyton density, 2) exhibit different temporal trends in assemblage composition, and 3) exhibit spatial differences in periphyton composition related to distance from the floodwater source.
Methods
Site Locations
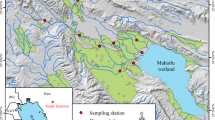
The study took place in Iberia and St. Martin Parishes, Louisiana, USA, and consisted of five ARB sites located in a 50-km2 section east of the Atchafalaya River (Fig. 1). The inlet of Bayou Postillion at the Gulf Intracoastal Waterway along the eastern Atchafalaya River Basin guide levee was designated as the source of Atchafalaya River water for the five sites. Five additional sites were located in Lake Verret (LV), a small, shallow wetland (SA = 56.98 km2, mean depth = 1.5 m) located on the east side of the AR. The sites located in the ARB and LV sites are approximately 20 km apart (the distance between the furthest sites is approximately 31 km). Both the ARB and LV are comprised of a complex network of natural and artificial channels and bayous and a few areas of shallow open water. The ARB and LV share a common climate and geomorphological origin, with both experiencing precipitation-driven flooding. Prior to the 1940’s, LV shared hydrologic connection with the AR and experienced annual flood pulses similar to other ARB floodplain areas. After construction of the protection levee by the US Army Corps of Engineers, LV no longer receives these annual pulses from the AR, but still experiences temporary pulses due to local precipitation events year-round (Report of the Chief of Engineers US Army 1941). During the spring, both the LV and ARB floodplains are inundated, with greater inundation in the ARB due to the combined river and precipitation-driven flooding, resulting in large expanses of inundated floodplains within both systems. Due to the differences in water sources, floods tend to recede earlier in LV than ARB. Within the ARB, floodwaters also carry substantial sediment loads, and the ARB floodplain is actively accreting, resulting in a loss of open water areas over time and an increase in coverage of seasonally inundated floodplain (Piazza 2014). Therefore, an additional consequence of loss of riverine connectivity between LV with ARB is a difference in the relative composition of permanently inundated bayous, canals, and open water areas.
Three periphyton samplers (periphytometers) were deployed at each of the five sites in LV and the ARB and tethered to trees on shore or on bald cypress (Taxodium distichum) knees in shallow water on the floodplain near the active shoreline (i.e., the shoreline defined by the current state of inundation). Sites were chosen mainly based on accessibility. In the ARB floodplain, channel width is highly variable, but channel width at the site locations generally did not exceed 15 or 20 m. Tree cover varied among sites and macrophyte density varied throughout the growing season. Sites were sampled biweekly from January 2019 – September 2019, although high water in the ARB precluded launching a boat to access sites in March and April.
Periphytometer Design
Periphytometers (15 × 30 cm) were constructed of 1-inch PVC pipe and sealed with water-resistant sealant, allowing the frame to float at the water surface. Four glass microscope slides (75 mm × 50 mm × 1 mm) were suspended along the length of the frame within approximately five centimeters from the surface of the water. Slides were secured with clear fishing line (4.5 kg test), and plastic clips spaced five centimeters apart to avoid loss or transfer of biofilm. Glass slides were cleaned thoroughly with ethanol prior to use and replaced with fresh slides biweekly.
Habitat and Water Quality Procedures
Sampling occurred for six months during 2019 at roughly the same time (± 1 h), which was important because algal chlorophyll expression changes throughout the day (Gargas et al. 1979; Owens et al. 1980). An AquaFluor Handheld Fluorometer (Turner Designs, San Jose, CA) was used to measure chlorophyl (CHL) and phycocyanin (PC) concentration of algal samples in situ at the time of collection (sterile, buffer dilution water was used as blank). Surface and bottom temperature measurements, dissolved oxygen (DO), specific conductance, pH, and turbidity were recorded at each site with a handheld YSI® multiprobe (Yellow Springs, OH). Water velocity was measured with a handheld velocimeter (SonTek®, YSI, Inc, Yellow Springs, OH). Tree cover and macrophyte cover were also recorded for each periphytometer. Tree cover was scored by a single observer as 0%, 20%, 40%, 80%, or 100%. To estimate macrophyte cover at the time of collection, a 75-cm × 75-cm frame was placed around each periphytometer and photographed from 1 m above. Images were used to estimate the percentage of floating or emergent plants surrounding each periphytometer.
To measure inorganic nutrients, water samples were collected in 1-L glass amber bottles that had been combusted at 550° C for 5 h to remove any residual carbon (rinsed twice with sample water and filled to the brim). Samples were immediately filtered through 0.45-μm pore filter, and spectrophotometry was used to determine nutrient concentration (Nitrite, NO2−N, Method 8507; Nitrate, NO3−N Method 8192; Phosphorus PO43−, Method 8048; Ammonia, NH3−N, Method 8155; APHA 2018).
To determine rates of respiration, which provides another estimate of algal density, microbial activity, and nutrient inputs (Rumschlag et al. 2020; Zhang et al. 2020), samples for 20-day biological oxygen demand (BOD; unfiltered sample) were collected in 1-Liter, opaque Nalgene bottles once per month at each collection site (rinsed twice with sample water and filled to brim before capping). Samples were stored on ice until processing. Prior to initial dissolved oxygen measurement, samples were raised to room temperature (20° C ± 2) and a nitrogen inhibitor was added. Dissolved oxygen measurements were taken every five days for 20 days; any bottle reading below 3.0 mg L−1 was bubbled with atmospheric oxygen for 5 min and re-measured before further incubation (APHA 2018).
Samples were also taken from algae scrapings to estimate heterotrophic bacterial abundance. One glass slide was removed, and a new, single-edge razor blade rinsed in 95% ethanol solution was used to scrape one-half of the slide into a sterile centrifuge vial filled with 50 mL of sterile, phosphate-buffered dilution water, put on ice for transportation back to the laboratory, and inoculated onto AR-2 agar for heterotrophic plate counts (HPC; APHA 2018). Plates were inverted and incubated at 35° C for 48 h prior to enumeration (APHA 2018) with a standard darkfield colony counter (Reichert Darkfield Quebec®, 220 V; Depew, NY).
To measure periphyton carbon and nitrogen content, a single periphytometer slide was placed (algae-side up) in an individual plastic box for transport. Samples were dried for 30 min at 60° C, scraped, weighed, and wrapped in tin capsules for processing. Carbon (total carbon) and nitrogen (total nitrogen) were measured by heating the tin/sample unit and measuring the gas products (N2 and CO2) from the combusted material via gas chromatography (Costech 1040 CHNOS Elemental Combustion, Valencia, CA; Matejovic 1993).
Algal Identification
Two periphytometer slides were collected biweekly and were placed in individual plastic bags with 10 mL of a 2% glutaraldehyde solution for algal identification. Glutaraldehyde preservative maintains cell color very well, which assists in taxonomic identification (Andersen 2005). The bags were refrigerated overnight so the glutaraldehyde would loosen the biofilm from the glass slide surface, reducing cell damage when scraped. Periphyton was scraped from the glass slides with a new, single-edge razor blade into centrifuge vials with additional 2% glutaraldehyde that completely covered the algae. Samples were refrigerated at 4° C in the dark. Extremely dense samples were diluted into 250 mL of glutaraldehyde. Prior to enumeration, samples were inverted gently to homogenize. If further homogenization was needed, the sample was sonicated for no more than 15 s. This was enough to break up dense clumps, but not enough to severely damage or burst a large number of cells. A Sedgewick-rafter counting slide observed at 400 × magnification (Leitz Laborlux K, Leica Microsytems, Wetzlar, Germany) was used to classify periphyton cells into 8 groups (cyanobacteria, centric diatoms, pennate diatoms, xanthophytes, euglenoids, chrysophytes, chlorophytes and unknown/other). Because of the high cell density, only a subset of cells in each sampled were counted and algae density was reported as cells/mm2.
Statistical Analysis
Descriptive analyses of periphyton composition were completed with JMP Pro (vers. 15.1.0, SAS Institute, Inc, Cary, NC), and all statistical analyses were performed with R (vers 3.6; R Core Team 2019) based on periphyton composition, as density and relative abundance, and measured environmental variables [nutrient concentrations, temperature, dissolved oxygen, pH, turbidity, water velocity, specific conductance, biochemical oxygen demand (BOD), colony-forming units (CFU), total organic carbon (TC), total nitrogen content (TN), carbon/nitrogen ratio (CN), chlorophyll a (CHL α), phycocyanin (PC), macrophyte density, tree cover (0–5 scale), site distance from the river water source, and river stage]. We used a log link-Poisson distribution general linear model (GLM; Vers. 3.6; R Core Team 2019) to analyze differences in total algal density between ARB and LV sites. To assess temporal and spatial trends in algal assemblage composition, Lake Verret and ARB data were analyzed independently with separate canonical correspondence analyses with a permutation test (n = 999) for included variables (CCA; R package vegan, Oksanen et al. 2019); the explanatory variable distance refers to the straight-line distance to river source for ARB sites and was excluded from the LV analysis. The CCA permitted identification of relationships between periphyton assemblage composition, based on density, and measured environmental variables. We chose CCA over other ordination methods because of the unimodal and constrained nature of the data (Palmer 2019). Components identified from the CCAs were then used in log link-Poisson distribution generalized linear models with the package lme4 (Vers. 3.6; R Core Team 2019) to further investigate trends in periphyton assemblage composition.
Results
Periphyton Assemblage
Periphytic algal density was significantly greater at ARB sites compared to LV sites (ARB: Mean = 2,358.12 cells/mm2, SE = 209.09; LV: Mean = 896.86 cells/mm2, SE = 41.99; P < 0.01, SE = 222.00) and overall community composition was also different between the two regions (Fig. 4). Periphyton assemblages in the ARB were dominated by diatoms in all months sampled. The relative abundance of centric diatoms was between 12–18% in January and February, then declined to less than 7% for the rest of the sampling period, whereas pennate diatom relative abundance was between 40–56% in all months. Chlorophytes usually did not exceed 30% of the total community assemblage. All other algal groups remained below 1%. In LV, the periphyton assemblage was dominated by chlorophytes in all months except January and February, when pennate diatoms were highest (61% and 68%, respectively). Centric diatoms never exceeded 3%. In both LV and ARB assemblages, cyanobacteria increased in the summer months, reaching over 20% in July.
Physicochemistry
The 2019 flood began on January 25 when the Atchafalaya River exceeded 3 m at the Butte la Rose (Gauge 07381515; Allen et al. 2008; Pasco et al. 2016) and ended on August 21, 2019. Sampling began on January 30 and continued biweekly until September 25, 2019, after the river entered the low-water stage (Fig. 2). LV did not experience spring flooding, but lake height and water velocity were influenced by local wind and rain events. In both ARB and LV sites (Table 1), dissolved oxygen (DO) showed higher concentrations early in the year, which declined in the summer months. In contrast, differences in the temporal patterns of nutrient concentrations were evident between ARB and LV sites. Nitrate (NO3) concentration was relatively low in LV compared to ARB sites, which exhibited higher nitrate values throughout the sampling period, particularly in January. Nitrite (NO2) concentrations were similar between the two sampling periods, whereas ammonium (NH4) was more variable in LV. Ammonium peaked twice in LV in May and September but was in low concentration during the other months. In the ARB, there was a single ammonium peak in February, with all other months exhibiting similar values. Temporal trends in phosphorus concentrations were similar in LV and ARB sites, although ARB sites exhibited lower concentrations in January and February. NP ratios indicated nitrogen limitation (ratio < 13; Hillebrand and Sommer 1999) in LV year-round and in the late spring and summer months in the ARB.
Daily Atchafalaya River stage obtained from the Butte la Rose water gauge (USGS 07381515) from Jan 2019 – Sep 2019. Horizontal line indicates flood stage (Pasco et al. 2016) and gray bar indicates sampling period
On average, both Total Carbon (TC) and Total Nitrogen (TN) were higher for Lake Verret relative to ARB sites, but both locations showed similar temporal concentration patterns, with peaks in TC and TN during July and lows in January. However, the CN ratio was slightly higher for ARB sites. Macrophyte cover was also higher in the ARB compared to LV (Figs. 3 and 4), although peak macrophyte cover occurred in the summer months at both locations and was dominated by floating taxa, primarily salvinia (Salvinia minima) and water hyacinth (Eichhornia crassipes). Overall, ARB sites located nearer to water sources supported higher macrophyte densities than sites located deeper in the floodplain.
Multivariate Analysis: ARB Sites
The first three components of the canonical correspondence analysis (CCA; Supplementary Table 1; Fig. 5a) explained the majority (99%) of variation in the data. Component 1 represented mostly temporal changes in periphyton assemblages (CCA1; 45% of the variation) and was positively associated with cyanobacteria and xanthophytes and a negatively associated with centric diatoms. Environmental variables positively associated with CCA1 included specific conductance, water temperature, sampling date, TC, TN, and phosphorus, with a negative association with DO. The second component represented primarily spatial features of environmental data and algal assemblages at ARB sites (CCA2; 42% of variation). Overall, total algal density was higher closer to river water input (β = -0.01, 0.01 SE, LR Chi = 2281.39, P < 0.01) with sites near the Gulf Intracoastal Waterway (GIWW) supporting higher cyanobacteria and centric diatom densities, greater ammonium concentrations, and higher velocity rates. Chlorophytes were lower in density closer to river inputs but increased in density further into the floodplain, where tree cover and community respiration (BOD) were higher. The last component (CCA3, variation 12%) represented the presence or absence of macrophytes. Sites heavy in aquatic vegetation tended to have higher pH levels and showed higher densities of both chrysophytes and euglenoids compared to sites with less macrophyte cover. Individual ARB sites (Fig. 5b) exhibited seasonal changes with respect to the environmental variables and were characterized by different algal groups over time. Permutation tests completed after the CCA (Table 2) indicated algal density was particularly influenced by sampling date, site, and DO, although all variables with the exception of water temperature, CHL, TC, tree cover, and macrophyte cover were related to periphyton density.
a Canonical correspondence analysis (CCA) plot for sites in the ARB. Variables include date (DAT), distance from water source (DIS), temperature (TMP), dissolved oxygen (DO), pH (PH), turbidity (TRB), water velocity (VEL), tree cover (TRC), macrophyte cover (MAC), Nitrate (NTA), Nitrite (NTI), biological oxygen demand (BOD), total carbon (TC), total nitrogen (TN), carbon/nitrogen ratio (CN), specific conductance (SPC), colony forming units (CFU), chlorophyll a (CHL), and phycocyanin (PC), chrysophytes (CHRY), centric diatoms (CENT), pennate diatoms (PENN), euglenoids (EUGL), chlorophytes (CHLO) xanthophytes (XANT), cyanobacteria (CYANO). Sites are S01 (dark green), S06 (light green), S08 (blue), Site09 (yellow), and S12 (red). b Arrows represent movement of sites across the CCA over time and were calculated by taking the mean of CCA values at each sampling date
General linear models for each algal group included only those variables that were significant in the permutation test, although included variables varied by algal group. Euglenoids (β = -937.10, 52080.00 SE), chrysophytes (β = 3.79, 17.40 SE), and xanthophytes (β = 3.79, 17.40 SE) did not show significant relationships for distance from source water, whereas cyanobacteria (β = -2.60, 1.05 SE), chlorophytes (β = -4.83, 1.47 SE) and centric (β = -170.10, 8.11 SE) and pennate diatoms (β = -14.78, -12.98 SE) did. Both pennate and centric diatoms showed negative estimates for distance, indicating that they were more abundant near river inputs. Centric and pennate diatoms also showed large positive relationships with nitrate (β = 13.62, 14.00 SE, respectively) and nitrite (β = 192.77, 194.63 SE, respectively). Chlorophytes showed positive estimates for only nitrite (β = 76.08, 78.14 SE).
Multivariate Analysis: Lake Verret Sites
Periphyton assemblages at LV sites differed substantially from sites located in the ARB. The first three components of the CCA explained 96% of the variability in the data (Supplementary Table 2; Fig. 6a). Similar to ARB sites, LV sites showed changes over time (Fig. 6b). The first component (CCA1; 73% of variation) described mostly seasonal variation in the data, with positive loadings for BOD, water velocity, and temperature, along with CFU, TC, and TN. Algal groups that also positively loaded on CCA1 were xanthophytes and cyanobacteria. Negative associations with CCA1 included pennate diatoms, DO, turbidity, CHL and CN ratio. The second component (CCA2; 19% variation) reflected areas that favored euglenoids, specifically sites high in phosphorus. The third component represented sites high in macrophyte cover (CCA3; 4% of variation) and was also positively related to chrysophytes, xanthophytes, centric diatoms, and euglenoid densities. The permutation test (Table 3) indicated that all the environmental variables were significant to periphytic algal assemblages except nitrate, nitrite, PC, and CN ratios. General linear models based on significant variables in the CCA indicated most taxa were more abundant earlier in the year, although sample date was not significant for all groups. Chrysophytes showed large positive associations with specific conductance (β = 12.62, 18.37 SE) whereas other groups showed no significant, or negative relationships. Pennate diatoms (β = -5.79, 0.16 SE) and xanthophytes (β = -3.87 ± 1.42 SE) were negatively related to water velocity, but centric diatoms (β = 6.25, 7.97 SE), chlorophytes (β = 2.16, 2.4 SE), and cyanobacteria (β = 2.16, 2.6 SE) were not.
Canonical correspondence analysis (CCA) for Lake Verret Sites. Variables include date (DAT), temperature (TMP), dissolved oxygen (DO), pH (PH), turbidity (TRB), water velocity (VEL), tree cover (TRC), macrophyte cover (MAC), nitrate (NTA), Nitrite (NTI), phosphorus (PHS), biological oxygen demand (BOD), total carbon (TC), total nitrogen (TN), carbon/nitrogen ratio (CN), specific conductance (SPC), colony forming units (CFU), chlorophyl (CHL), and phycocyanin (PC). Sites are V01 (dark green), V02 (light green), V03 (blue), V04 (yellow), and V05 (red). b. Arrows represent movement of sites across the CCA over time and were calculated by taking the mean of CCA values at each sampling date
Discussion
In our study, periphyton assemblages in both sampling regions showed temporal variation and were impacted by several environmental factors, including temperature, nutrients, and macrophyte cover. Assemblages in the ARB were additionally influenced by distance from water source. Importantly, changes to historical flooding regimes in the ARB and other floodplain rivers that limit distance penetrated by floodwaters or create full disconnection could significantly alter floodplain productivity, and river-floodplain connectivity associated relationships to the structure of floodplain trophic webs should be considered in the management of floodplain river systems.
Temporal Effects on Algal Assemblages
Both ARB and LV sites showed seasonal variation in measured environmental variables, which impacted the dynamics of the algal groups. For ARB sites, CCA1 represented temporal trends in periphyton composition. In this study, due to its temporal length, the annual flood-pulse occurs concurrently with seasons (i.e., the rising limb occurs through the spring and early summer and the falling limb occurs mid to late summer). Therefore, separating the flood impact from seasonal changes is not possible. However, temporal changes are relatively predictable in river-floodplain systems (e.g., Kaller et al. 2011; Pasco et al. 2016; Kroes et al. 2022). High water turbidity and increases in DO concentration and periphyton CN ratio indicated periods of floodplain inundation, which typically occurs in the early months of the year depending on the timing and magnitude of the Atchafalaya River flood pulse (Kaller et al. 2011). Later in the season, usually in May and June, Atchafalaya River stages decline, and inundated floodplains drain into canals and bayous, eventually entering the Atchafalaya River to the west or the GIWW to the east. Temporal changes in periphyton composition were also evident in LV but were not related to seasonal rising and falling water levels. The early part of the year for these sites was characterized by increased DO levels and high chlorophyll-a concentrations. As temperatures increased later in the year, there was an increase in algal and bacterial growth, as indicated by TN correlations, as well as respiration rates (BOD) and microbial associations (CFU). Although LV does slowly drain into Grassy Lake, directional north-to-south velocity is negligible, and water movement is largely due to local wind action. Interestingly, cyanobacteria were highly correlated with increased water movement in Lake Verret, even though cyanobacteria in lotic systems have been reported to prefer little to no water movement (Bellinger and Sigee 2015; Pacheco and Neto 2017). Most likely, greater densities of cyanobacteria later in the year were more related to elevated temperatures than the influence of increased water movement, as seen in other freshwater lake systems (Beaulieu et al. 2013; Mullin et al. 2020).
Pennate diatoms were largely ubiquitous at ARB sites, as in other riverine ecosystems (Finlay et al. 2002) and did not appear on any CCA components, although centric diatoms were associated with CCA1 and CCA2. In LV, diatoms, particularly pennate diatoms, significantly declined during the warmer parts of the year. This is similar to observations from another group of floodplain lakes in the Yangtze River system. There, diatoms dominated phytoplankton assemblages in lakes with active river connections, such as the ARB. However, unlike the LV results where chlorophytes were the dominant taxa, isolated lakes in the Yangtze River floodplain were dominated by cyanobacteria (Liu et al. 2017), which might have been related to higher nutrient concentrations in the isolated systems. In ARB and LV, diatoms were closely associated with turbidity and high DO, characteristic of ARB flooding conditions and extensive rainfall in both locations early in the year. Although high levels of turbidity generally reduce photosynthetic activity by blocking incoming light (Bellinger and Sigee 2015), it may be that turbidity levels at this time, although elevated relative to other parts of the year, were not high enough to impact photosynthesis on the shallowly suspended (5 cm) periphytometers. In addition, diatoms can be highly sensitive to low DO levels (Szczepocka et al. 2018), which are highest during river rising events when water temperature is low, and may provide optimal conditions for periphyton growth, at least near the water surface. Interestingly, diatoms typically have high TC and TN content and are a valued consumer resource (Brett et al. 2009; Guo et al. 2016). Therefore, we expected they would have loaded with TC and TN on the CCA axes. Potentially, uptake from the diatoms themselves had depleted TC and TN prior to sampling, but this seems unlikely, given the high levels of TC and TN in the ARB (Whitall 2008). Prior to elemental analysis, periphyton growth slides were viewed under a dissecting microscope and large macroinvertebrates, such as chironomid larvae, were removed. However, smaller grazers, like Cladocera and rotifers, may have been included and confounded the analyses.
Xanthophytes also varied temporally in their contribution to the periphyton assemblages at both locations. Xanthophytes can be single-celled or colonial and appear yellow-green in color due to the accessory pigment diatoxanthin (Bellinger and Sigee 2015). Members of this algal group, such as Botrydiopsis arrhizal, are commonly found in muddy habitats near littoral edges (Bellinger and Sigee 2015; Reynolds 2006), in small water bodies, or in soil (Reynolds 2006; Bellinger and Sigee 2015; Zhang et al. 2015; Costa et al. 2020). In both regions, this group made up less than 1 percent of the periphytic algal assemblage but exhibited its highest abundances in the late summer months in both regions. Xanthophytes prefer cool, free-standing, slightly acid water (Gabyshev and Gabysheva 2010), therefore, their appearance in Lake Verret later in the season was somewhat unexpected. However, Reynolds (2006) reported xanthophyte abundance could be driven by low turbidity levels, which may explain their loadings on CCA1.
Distance from River Source Impacts Algal Assemblages
Although temporal influences were important determinants of algal community structure, there were also significant spatial effects on periphyton assemblage composition in the ARB. Areas closer to river water input showed biotic and abiotic trends that differed from areas deeper within the floodplain. As the floodplain becomes inundated, rising water crests natural levees and begins to move onto the floodplain. Water velocity increases differentially as river water rises, with sites nearer river water sources showing higher water velocities than more distant sites. Dry floodplain areas with accumulated organic matter during low-water are inundated with nitrogen-rich river water, while hydrologic mixing occurs in connected floodplain lakes (Kaller et al. 2015; Vargas-Lopez et al. 2020; Kroes et al. 2022). Newly available nutrients (NO3, NO2, NH4) fuel algal and bacterial growth. In the ARB sites, nitrogen and phosphorus sources were inversely related to distance from the river source and would have been readily taken up by early colonizers, such as diatoms. In rivers and lakes, diatoms, particularly large centric diatoms, are typically the first to exploit influxes of nutrients (Brett et al. 2009; Dai et al. 2012; Bellinger and Sigee 2015; Reynolds 2002; Kiss et al. 2012), explaining why centric diatoms and water nutrients were related on the CCA. Chlorophytes, in contrast, did not exhibit substantial seasonal trends observed in other riverine and lake systems (Sheath and Burkholder 1983; Andersen et al. 2020), but did appear to be more abundant at sites located further from river sources, where inorganic nitrogen was lower. In the ARB, back-water swamps are characterized by high amounts of canopy cover and decomposition due to microbial activity (BOD; Battle and Mihuc 2000). Greater chlorophyte abundance at more distant sites was unexpected given their requirements for high light intensity and low shade tolerance (Lemes-da-Silva et al. 2010; Tonetto et al. 2012; Peres et al. 2017). However, it is likely that chlorophytes were simply more abundant later in the year when turbidity had declined, and light levels were sufficient for photosynthesis, regardless of shading. This is also supported by their association with BOD, which was also higher later in the year when water temperatures and community respiration increased. The magnitude and duration of floodplain inundation can vary substantially in the ARB given annual variability in the Atchafalaya River flood pulse (e.g., Pasco et al. 2016). In 2019, the river remained in flood stage until nearly September. This prolonged inundation, particularly in the backwater regions most distant from river inputs, could have provided particularly suitable environmental conditions for chlorophyte growth for an extended period relative to more typical flood years (i.e., the more rapid decline in water quality when cooler water is not present as long in the year; Kroes et al. 2022).
Macrophytes
Native macrophytes are essential to aquatic ecosystems because they influence habitat and water quality, which determines organism abundance and distribution (Caraco and Cole 2002; Dodds and Biggs 2002; Kaller et al. 2011; Pasco et al. 2016), as well as also serving as substrate for basal resource development (Cazzanelli et al. 2021). As such, in both ARB and LV, macrophyte cover (CCA3) appeared influential in driving periphytic algal assemblages, but was only significant in LV. Specifically, chrysophytes, euglenoids, and xanthophytes in LV sites were positively associated with macrophytes. These taxa are generally present in small numbers but can become dominant under favorable conditions. Chrysophytes, which can be unicellular, colonial, or filamentous (Reynolds 2006), tend to be found in cooler, oligotrophic waters that are low pH, specific conductance, and alkalinity. There have been very few observations of freshwater chrysophytes associated with macrophytes, therefore their correlation with aquatic macrophytes in LV was unexpected (Siver and Hamer 1989; Siver and Hamer 1992; Bellinger and Sigee 2015). However, Chrysomorula choaerens has been observed previously to form dense colonies on aquatic macrophytes (Wujek 2013), and Tunca et al. (2014) found several taxa in northern Turkey that commonly occurred with aquatic macrophytes (i.e. Chromulina sp., Ochromonas sp. Psuedokephyrion sp.). In 2018, Cao and colleagues noted that when P was abundant in heavily vegetated ponds, algal communities tended to be dominated by chrysophytes, rather than cyanobacteria, which were usually most abundant (Cao et al. 2018). More studies are needed to better understand the occurrence of chrysophytes in disconnected floodplain lakes like LV, as the current study did not identify chrysophytes to genera or species. Xanthophytes also generally constitute only a small portion of the periphytic community, and similarly to chrysophytes, are rarely found among aquatic plants. The few occurrences that have been observed tended to be filamentous (Trebonema sp.) or coccoid (Mischococcus sp.; Ott and Oldham-Ott 2003; Salmaso and Tolotti 2009). It was not unexpected to observe euglenoids among macrophyte beds in LV, as they are far more common among aquatic vegetation and can be found in small ponds or areas with high amounts of decaying organic matter (Bellinger and Sigee 2015; Wehr et al. 2015; Cao et al. 2018), particularly those dominated by submerged macrophytes (Dokulil and Padisak 1994).. Moreover, it has been reported that when macrophyte abundance exceeded 40%, euglenoids consistently dominated algal assemblages (Borics et al. 2003). One of the reasons why euglenoids can thrive in vegetated areas, where competition for light and nutrients is high, is because of their mixotrophic strategies that allow proliferation in resource-limiting environments. Mixotrophy is also common xanthophytes and chrysophytes, so it is possible that this strategy is what allowed them to thrive in competitive environments with euglenoids in macrophyte-dominated habitats (Tunca et al. 2014; Pribyl and Cepak 2019).
Conclusions
Alterations to river-floodplain systems threatens habitat integrity and can contribute to the loss of ecosystem function and biodiversity. Disconnection of floodplain lakes from their rivers by installing dams and levees (as seen in Lake Verret) causes changes in hydrology that can significantly alter macrophyte and fish assemblage composition and function (Liu and Wang 2010; Quirino et al. 2019; Jiang et al. 2020). Periphytic algal assemblages in the ARB differed from LV, particularly in overall higher cell abundance, pennate diatom-dominated assemblages, a seasonal shift from pennate diatoms to chlorophytes, and a gradient of decreasing overall cell abundance and a shift from centric diatoms to chlorophytes at increasing distances into the floodplain. In contrast, in LV samples, pennate diatoms were replaced by cyanobacteria and xanthophytes and in the absence of a floodplain gradient, assemblages were similar among sites. However, in both systems, vegetated areas were regularly associated with euglenoids, chrysophytes, and xanthophytes (only LV). Importantly, distance from the water source had a substantial effect on both algal community composition and environmental variables in the ARB floodplain. Overall cell abundance decreased further into the floodplain, with centric diatoms showing higher prevalence closer to river water sources, characterized by greater velocity and nitrogen concentrations. Deeper into floodplain habitats, tree cover and microbial activity were greater, favoring chlorophytes. Because shifts in the composition of basal resources could impact higher trophic levels, changes to historical flooding regimes and their impact on floodplain trophic webs should be considered in future floodplain management decisions.
Data Availability
The data sets for this study are not available.
References
Adame MF, Pettit NE, Valdez D, Ward D, Burford MA, Bunn SE (2017) The contribution of epiphyton to the primary production of tropical floodplain wetlands. Biotrophica 49:461–471
Agostinho AA, Gomes LC, Verissimo S, Okada EK (2004) Flood regime, dam regulation and fish in the Upper Parana River: effects on assemblage attributes, reproduction and recruitment. Reviews in Fish Biology and Fisheries 14:11–19
Agostinho AA, Pelicice FM, Gomes LC (2008) Dams and fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Brazilian Journal of Biology 68:1119–1132
Ahearn DS, Viers JH, Mount JF, Dahlgren RA (2006) Priming the productivity pump: flood pulse driven trends in suspended algal biomass distribution across a restored floodplain. Freshwater Biology 51:1417–1433
Algarte VM, Dunck B, Leandrini JA, Rodrigues L (2016) Periphytic diatom ecological guilds in floodplain: Ten years after dam. Ecological Indicators 69:407–414
Allen YC, Constant GC, Couvillion BR (2008) Preliminary classification of water areas within the Atchafalaya Basin Floodway system by using Landsat imagery. U.S. Geological Survey Open-File Report 2008–1320, p 14
Andersen RA (2005) Algal culturing techniques. Elsevier, Burlington MA
Andersen IM, Williamson TJ, Gonzalez MJ, Vanni MJ (2020) Nitrate, ammonium, and phosphorus drive seasonal nutrient limitation of chlorophytes, cyanobacteria, and diatoms in a hyper-eutrophic reservoir. Limnology and Oceanography 65:962–978
APHA. American Public Health Association (2018) Standard methods for the examination of water and wastewater, 20th edn. Am. Publ. Hlth. Ass, New York
Battin TJ, Besemer K, Bengtsson MM, Romani AM, Packmann AI (2016) The ecology and biogeochemistry of stream biofilms. Nature Reviews Microbiology 14:251–263
Battle JM, Mihuc TB (2000) Decomposition dynamics of aquatic macrophytes in the lower Atchafalaya, a large floodplain river. Hydrobiologia 418:13–136
Bayley PB, Castello L, Batista VS, Fabre NN (2018) Response of Prochilodus nigricans to flood pulse variation in the central Amazon. Royal Society Open Science 5:172232
Beaulieu M, Pick F, Gregory-Eaves I (2013) Nutrients and water temperature are significant predictors of cyanobacterial biomass in a 1147 lakes data set. Limnological Oceanography 58:1736–1746
Beisner BE, Peres-Neto PR, Lindstrom ES, Barnett A, Longhi ML (2006) The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology 87:2985–2991
Bellinger EG, Sigee DC (2015) Freshwater algae: Identification, enumeration, and use as bioindicators. 2nd edn. Wiley, Hobokin NJ
Borics G, Tothmeresz B, Grigorszky I, Padisak J, Varbiro G, Szabo S (2003) Algal assemblage types of bog-lakes in Hungary and their relation to water chemistry, hydrological conditions and habitat diversity. Hydrobiologia 502:145–155
Bortolini JC, Train S, Rodrigues LC (2016) Extreme hydrological periods: effects on phytoplankton variability and persistence in a subtropical floodplain. Hydrobiologia 763:223–236
Brett MT, Kalnz MJ, Talpale SJ, Seshan H (2009) Phytloplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proceedings of the National Academy of Sciences 106:21197–21201
Campos-Silva JV, Peres CA, Amaral JHF, Sarmento H, Forsberg B, Fonseca CR (2021) Fisheries management influences phytoplankton biomass of Amazonian floodplain lakes. Journal of Applied Ecology 58:731–743
Cao Y, Zhang N, Sun J, Li W (2018) Responses of periphyton on non-plant substrates to different macrophytes under various nitrogen concentrations: A mesocosm study. Aquatic Botany 154:53–59
Caraco N, Cole JJ (2002) Contrasting impacts of native and alien macrophyte on dissolved oxygen in a large river. Ecological Applications 12:1496–1509
Cazzanelli M, Soria-Barreto M, Castillo MM, Rodiles-Hernandez R (2021) Seasonal variations in food web dynamics of floodplain lakes with contrasting hydrological connectivity in the Southern Gulf of Mexico. Hydrobiologia 848:773–797
Colon-Gaud J, Kelso WE, Rutherford DA (2004) Spatial distribution of macroinvertebrates inhabiting hydrilla and coontail beds in the Atchafalaya Basin, Louisiana. Journal of Aquatic Plant Management 42:85–91
Costa APT, Crossetti LO, Hartz SM, Becker FG, Hepp LU, Bohnenberger JE, Lima MS, Guimaraes T, Schneck F (2020) Land cover is the main correlate of phytoplankton beta diversity in subtropical coastal shallow lakes. Aquatic Ecology 54:1015–1025
Crook DA, Buckle DJ, Morrongiello JR, Allsop QA, Baldwin W, Saunders TM, Douglas MM (2019) Tracking the resources pulse: Movement responses of fish to dynamic floodplain habitat in a tropical river. Journal of Animal Ecology 89:795–807
Dai G, Shang J, Qiu B (2012) Ammonia may play an important role in the succession of cyanobacterial blooms and the distribution of common algal species in shallow freshwater lakes. Glob Change Biol 18:1571–1581
Dodds WK, Biggs BJ (2002) Water velocity attenuation by stream periphyton and macrophytes in relation to growth form and architecture. Journal of the North American Benthological Society 21:2–15
Dokulil MT, Padisak J (1994) Long-term compositional response of phytoplankton in shallow, turbid environment, Neusiedlersee (Austria/Hungary). Hydrobiologia 276:125–137
Fernandes R, Agostinho AA, Ferreira EA, Pavanelli CS, Suzuki HI, Lima DP, Gomes LC (2009) Effects of the hydrological regime on the ichthyofauna of riverine environments of the Upper Parana River floodplain. Brazilian Journal of Biology 69:669–680
Finlay BJ, Monaghan EB, Maberly SC (2002) Hypothesis: The rate of scale of dispersal of freshwater diatom species is a function of their global abundance. Protist 153:261–273
Fisher JC, Kelso WE, Rutherford DA (2012) Macrophyte mediated predation on hydrilla-dwelling macroinvertebrates. Fundamental Applied Limnology 181:25–38
Flemming H (1993) Biofilms and environmental protection. Water Science Technology 27:1–10
Ford M, Nyman JA (2011) Preface: an overview of the Atchafalaya River. Hydrobiologia 658:1–5
Gabyshev VA, Gabysheva OI (2010) Water quality of the Anabar River indicated by phytoplankton structure and hydrochemical characteristics. Contemporary Problems of Ecology 3:395–400
Gargas E, Hare I, Martens P, Edler L (1979) Diel changes in phytoplankton photosynthetic efficiency in brackish waters. Marine Biology 52:113–122
Guo F, Kainz MJ, Sheldon F, Bunn SE (2016) Effects of light and nutrients on periphyton and the fatty acid composition and somatic growth of invertebrate grazers in subtropical streams. Oecologia 181:449–462
Hillebrand H, Sommer U (1999) The nutrient stoichiometry of benthic microalgae growth: Redfield proportions are optimal. Limnol Oceanogr 44:440–446
Jardine TD, Pettit NE, Warfe DM, Pusey BJ, Ward DP, Douglas MM, Davies PM, Bunn SE (2012) Consumer-resource coupling in wet-dry tropical rivers. Journal of Animal Ecology 81:310–322
Jardine TD, Bond NR, Burford MA, Kennard MJ, Ward DP, Bayliss P, Davies PM, Douglas MM, Hamilton SK, Melack JM, Naiman RJ, Pettit NE, Pusey BJ, Warfe DM, Bunn SE (2015) Does flood rhythm drive ecosystem response in tropical riverscape. Ecology 96:684–692
Jiang X, Pan B, Sun Z, Lu Y (2020) Application of taxonomic distinctness indices of fish assemblages for assessing effects of river-lake disconnection and eutrophication in floodplain lakes. Ecological Indicators 110:105955
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river -floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences 106:110–127
Kalff J (2002) Phytoplankton. Limnology: Inland water ecosystems. Prentice Hall, Upper Saddle River, NJ, pp 309–348
Kaller MD, Kelso WE, Halloran BT, Rutherford DA (2011) Effects of spatial scale on the assessment of dissolved oxygen dynamics in the Atchafalaya River Basin, Louisiana. Hydrobiologia 658:7–15
Kaller MD, Keim RF, Edwards BL, Harlan AR, Pasco TE, Kelso WE, Rutherford DA (2015) Aquatic vegetation mediates the relationship between hydrologic connectivity and water quality in a managed floodplain. Hydrobiologia 790:29–41
Kiss KT, Klee R, Ector L, Acs E (2012) Centric diatoms of large rivers and distributaries in Hungary: morphology and biogeographic distribution. Acta Botanica Croatica 71:311–363
Kroes DE, Demas CR, Allen YA, Day RH, Roberts SW, Varisco J (2022) Hydologic modifcation and channel evolution degrades connectivity on the Atchafalaya River floodplain. Earth Surf Process Landf 47:1790–1807. https://doi.org/10.1002/esp.5347
Lansac-Toha FM, Meira BR, Segovia BT, Lansac-Toha FA, Velho LFM (2016) Hydrological connectivity determining metacommunity structure of plankton heterotrophic flagellates. Hydrobiologia 78:81–94
Lemes-da-Silva NM, Branco LHZ, Necchi-Junior O (2010) Corticolous green algae from tropical forest remnants in the northwest region of Sao Paulo State, Brazil. Brazilian Journal of Botany 33:215–226
Lewis WM, Hamilton SK, Lasi MA, Rodriguez M (2000) Ecological determinism on the Orinoco Floodplain: A 15-year study of the Orinoco floodplain shows that this productive and biotically diverse ecosystem is functionally less complex than it appears. Hydrographic and geomorphic controls induce a high degree of determinism in biogeochemical and biotic processes. Bioscience 50:681–692
Li C, Feng W, Chen H, Li X, Song F, Guo W, Giesy PJ, Sun F (2019) Temporal variation in zooplankton and phytoplankton, community species composition and the affecting factors in Lake Taihu - a large freshwater lake in China. Environmental Pollution 245:1050–1057
Liboriussen L, Jeppesen D (2006) Structure, biomass, production, and depth distribution or periphyton on artificial substratum in shallow lakes with contrasting nutrient concentration. Freshwater Biology 51:95–109
Liu X, Wang H (2010) Estimation of minimum area requirement of river-connected lakes for fish diversity conservation in the Yangtze River floodplain. Diversity and Distributions 16:932–940
Liu X, Qian K, Chen Y, Gao J (2017) A comparison of factors influencing the summer phytoplankton biomass in China’s three largest freshwater lakes: Poyang, Dongting, and Taihu. Hydrobiologia 792:283–302. https://doi.org/10.1007/s10750-016-3063-5
Matejovic I (1993) Determination of carbon, hydrogen, and nitrogen in soils by automated elemental analysis (dry combustion method). Communications in Soil Science and Plant Analysis 24:17–18
Mazumder D, Saintilan N, Wen L, Kobayashi T (2017) Productivity influences trophic structure in a temporally forced aquatic ecosystem. Freshwater Biology 62:1528–1538. https://doi.org/10.1111/fwb.12963
Mullin CA, Kirchhoff CJ, Wang G, Vlahos P (2020) Future projections of water temperature and thermal stratification in Connecticut reservoirs and possible implications for cyanobacteria. Water Resources Research 56:e2020WR027185
NOAA Fisheries: Office of Science and Technology, Annual Commercial Landing Statistics. (2018) NOAA: Silver Springs
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH (2019) Package vegan. R package version 2.5–6
Opperman JJ, Luster R, McKenny BA, Roberts M, Meadows AW (2010) Ecologically functional floodplains: Connectivity, flow regime, and scale. Journal of the American Water Resources Association 46(2):211
Ott DW, Oldham-Ott CK (2003) Eustigmatophyte, Rhodophyte, and Tribophyte Algae. Wehr, J.D. and Sheath 424–427
Owens TG, Falkowski PG, Whitledge TE (1980) Diel periodicity in cellular chlorophyll content in marine diatoms. Marine Biology 59:71–77
Pacheco CHA, Neto IEL (2017) Effects of artificial circulation on the removal kinetics of cyanobacteria in a hypereutrophic shallow lake. Journal of Environmental Engineering 143:06017010
Palmer MW (2019) Gradient analysis of ecological communities (ordination). In: Gelfund A, Fuentes M, Hoeting JA, Smith RL (eds) Handbook of environmental and ecological statistics. CRC Press, Boca Raton, pp 214–274
Pasco TE, Kaller MD, Harlan R, Kelso WE, Rutherford DA (2016) Predicting floodplain hypoxia in the Atchafalaya River, Louisiana, USA, a large, regulated southern floodplain river system. River Research and Applications 32:845–855
Peres CK, Tonetto AF, Garey MV, Branco CCV (2017) Canopy cover as the key factor for occurrence and species richness of subtropical stream green algae (Chlorophyta). Aquatic Botany 137:24–29
Pettit NE, Naiman RJ, Warfe DM, Jardine TD, Douglas MM, Bunn SE, Davies PM (2017) Productivity and connectivity in tropical riverscapes of Northern Australia: Ecological insights for management. Ecosystems 20:492–514
Piazza BP (2014) The Atchafalaya River Basin: history and ecology of an American wetland. No. 26. Texas A&M University Press
Power ME, Bouma-Gregson K, Higgins P, Carlson SM (2015) The Thirsty Eel: Summer and winter flow threshold that tilt the Eel River of Northwest California from salmon-supporting to cyanobacterially degraded stats. Copeia 1:200–211
Pribyl P, Cepak V (2019) Screening for heterotrophy in microalgae of various taxonomic positions and potential of mixotrophy for production of high-value compounds. Journal of Applied Phycology 31:1555–1564
Quirino BA, Carniatto N, Thomaz SM, Fugi R (2019) Small fish diet in connected and isolated lakes in a Neotropical floodplain. Ecol Freshw Fish 28:97–109
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Report of the Chief of Engineers, U.S. Army (1941) Annual Reports, War Department. United States Government Printing Office, Washington
Reynolds CS (1994) The long, the short and the stalled: n the attributes of phytoplankton selected by physical mixing in lakes and rivers. Hydrobiologia 289:9–21
Reynolds CS (2002) Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24:417–428
Reynolds CS (2006) The ecology of phytoplankton. Cambridge University Press, Cambridge
Rumschlag SL, Mahon MB, Hoverman JT, Raffel R, Carrick HJ, Hudson PJ, Rohr JR (2020) Consistent effects of pesticide on community structure and ecosystem function in freshwater systems. Nature Communications 11:1–9
Rutherford DA, Gelwicks KR, Kelso WE (2001) Physicochemical effects of the flood pulse on fishes in the Atchafalaya River Basin, Louisiana. Transactions of the American Fisheries Society 130:276–288
Sabo M, Bryan CF, Kelso WE, Rutherford DA (1999) Hydrology and aquatic habitat characteristics of a riverine swamp: II Hydrology and the occurrence of chronic hypoxia. Regulated Rivers: Research and Management: an International Journal Devoted to River Research and Management 15:525–544
Salmaso N, Tolotti M (2009) Other phytoflagellates and groups of lesser importance. Elsevier, Trento, Italy, pp 174–183
Sheath RG, Burkholder JM (1983) Morphometry of Batrachospermum populations intermediate between B. boryanum and B. ectocarpu (Rhodophyta). Journal of Phycology 19:324–331
Shurin JB, Cottenie K, Hillebrand H (2009) Spatial autocorrelation and dispersal limitation in freshwater organisms. Oecologia 159:151–159
Siver PA, Hamer JS (1989) Multivariate statistical analysis of the factors controlling the distribution of scaled chrysophytes. Limnology and oceanography 34:368–381
Siver PA, Hamer JS (1992) Seasonal periodicity of chrysophytes and synurophyceae in a small New England Lake: Implications for paleolimnological research. Journal of Phycology 28:186–198
Sokal MA, Hall RI, Wolfe BB (2010) The role of flooding on inter-annual and seasonal variability of lake water chemistry, phytoplankton diatom communities and macrophyte biomass in the Slave River Delta (Northwest Territories Canada). Ecohydrology 3:14–54
Szczepocka E, Nowicka-Krawczyk P, Kruk A (2018) Deceptive ecological status of urban streams and rivers – evidence from diatom indices. Ecosphere 198:113800
Tonetto AF, Branco CCZ, Peres CK (2012) Effects of irradiance and spectral composition on the establishment of macroalgae in streams in southern Brazil. Annales de Limnologie-International Journal of Limnology 48:363–370
Troutman JP, Rutherford DA, Kelso WE (2007) Patterns of habitat use among vegetation-dwelling littoral fishes in the Atchafalaya River Basin, Louisiana. Transactions of the American Fisheries Society 136:1063–1075
Tunca H, Ongun Sevindik T, Bal DN, Arabaci S (2014) Community structure of epiphytic algae on three different macrophytes at Acarlar floodplain forest (northern Turkey). Chinese Journal of Oceanology and Limnology 32:845–857
Vargas-Lopez IA, Kelso WE, Bonvillain CP, Keim RF, Kaller MD (2020) Influence of water quality, local knowledge and river-floodplain connectivity on commercial wild crayfish harvesting in the Atchafalaya River Basin. Fisheries Management and Ecology. https://doi.org/10.1111/fme.12422
Walley RC (2007) Environmental factors affecting the distribution of native and invasive aquatic plants in the Atchafalaya River Basin, Louisiana, USA
Wehr JD, Sheath RG, Kociolek JP (eds) (2015) Freshwater algae of North America: ecology and classification. Elsevier, Walthem MA
Wetzel RG (1964) This week’s citation classic. Internationale Revue der gesamten Hydrobiologia 49:1–61
Wetzel RG (1983) Attached algal-substrata interactions: Fact or myth, and when and how? In: Wetzel R (ed) Periphyton of Freshwater Ecosystems, vol 17. Springer, Neatherlands, pp 207–215
Whitall DR (2008) Historical nitrogen and phosphorus loadings in the northern Gulf of Mexico. NOS NCCOS 85, NOAA/NOS/Center for Coastal Monitoring and Assessment, Silver Spring
Wujek DE (2013) A new freshwater chrysophyte, Chrysomorula cohaerens gen. et sp. nov. (Chrysophyceae, Chrysocapsaceae) from North America. Phytotaxa 93:61–64
Zhang M, Xu J, Hansson LA (2015) Local environment overrides regional climate influence on regime shift in a north temperate lake. Aquatic Ecology 49:105–113
Zhang J, Shu X, Zhang Y, Tan X, Zhang Q (2020) The response of epilithic algal community structure and function to light and nutrients and their linkages in subtropical rivers. Hydrobiologia 847:841–855
Funding
This work was supported by the US Army Corp or Engineers (Grant Number MIPR W42HEM90940923). Partial support for this project came from the U.S. Department of Agriculture National Institute of Food and Agriculture, Hatch Program as Project Number LAB-94410 and McIntire-Stennis Cooperative Forestry Program as Project Number LAB-94335. This manuscript was approved for publication by the Director of the Louisiana Agricultural Experiment Station as MS 000–000-00000.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study concept and design. Field sampling was done by Dr. Kamela Gallardo. Drs. Kelso and Rutherford had contributed their extensive knowledge of river-floodplain systems. Dr. Reagan Errera contributed substantially to this project with her extensive knowledge of freshwater algae. Special thanks should be given to Dr. Michael Kaller for his help with statistical analysis. All authors have approved this manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Gallardo, K., Kaller, M.D., Rutherford, D.A. et al. Influence of River Disconnection on Floodplain Periphyton Assemblages. Wetlands 43, 23 (2023). https://doi.org/10.1007/s13157-023-01668-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-023-01668-5