Abstract
Biological invasions cause species extinction but can also provide benefits. Wetlands, such as salt marshes, include little-known but important ecosystems that are sometimes severely invaded by exotic plants. Salt marshes in eastern South America are increasingly impacted by invasions of the African grass Urochloa arrecta. This study investigated the appearance of a population of the mangrove rail Rallus longirostris in areas dominated by U. arrecta and its disappearance with the eradication of this plant. We monitored four areas (54.47 ha) in the Guaratuba Bay estuary in southern Brazil, from 2006 to 2022, two of which contained four patches of U. arrecta as the dominant species. In 2012, we started to eradicate U. arrecta with mechanical management, and in 2020, it was eradicated locally. We recorded R. longirostris for the first time within a patch of U. arrecta in 2007. In subsequent years we saw the species in two other patches of the exotic plant. Rallus longirostris was no longer observed once U. arrecta was eradicated. Differences in patch occupancy between invaded and uninvaded habitats observed for R. longirostris and Pardirallus nigricans, and the disappearance of R. longirostris following the exotic plant management suggest competitive advantage and/or differential habitat preference and population density as hypotheses to explain observed patterns. The invasion of U. arrecta can increase the local populations of R. longirostris. Since this bird is not endangered, we encourage the management of U. arrecta because of its impact on salt marshes, including an endemic endangered bird.
Similar content being viewed by others
Introduction
The introduction of alien species has intensified with globalization in recent decades (Meyerson and Mooney 2007). Exotic species are defined as species that are not native to an ecosystem and that cause or are likely to cause economic, environmental, and/or human health damage (Catling 2005). They can change the composition of ecosystems rapidly and profoundly (Hobbs et al. 2009) and, through their direct and indirect effects, contribute substantially to species extinction (Vitousek et al. 1997; Bellard et al. 2016). Consequently, biological invasions are considered the second most common cause of biodiversity loss (Simberloff 2007). However, the effects of invasive species are not all negative, and the “native good/alien bad” dichotomy has been questioned (Goodenough 2010). Exotic species can benefit native species through habitat modification, trophic subsidy, pollination, competitive release, and predator release mechanisms (Lees and Bell 2008; Overton et al. 2014), besides having educational potential (Battisti 2016; Battisti et al. 2018). Comprehending and studying the responses of native species to invasive alien species and their management is essential for understanding impacts and deciding conservation actions (Schlaepfer et al. 2002; Lees and Bell 2008).
Wetland ecosystems can be greatly disturbed by biological invasions (Levin et al. 2006; Reinert et al. 2007; Norbdy et al. 2009; Cuassolo et al. 2012). These environments are vital, and despite covering only 6% of the Earth’s surface, they host 24% of the most invasive species on the planet (Zedler and Kercher 2004). Salt marshes, as a type of wetland, are possibly the most important and least understood of the world’s major ecosystems (Gedan et al. 2011). They are dynamic coastal areas (Watson and Byrne 2009; Gedan et al. 2011) that host salt-tolerant plant species (Doody 2001). They are regularly flooded by tides, have rapid sediment accumulation, and include transitions to non-tidal vegetation in the absence of human interference (Doody 2001). They occur in temperate areas across the globe, are more extensive in the northern hemisphere, and support seagrass Spartina spp. as the most common plant species (Doody 2001). Recently, some marshes on the South American Atlantic coast have been recognized as salt marshes—specifically subtropical salt marshes (Bornschein et al. 2017). They are characterized by the dominance of the crinum lily Crinum americanum L. and the California bulrush Schoenoplectus californicus (C. A. Mey.) Soják; therefore, the presence of smooth cordgrass Spartina alterniflora Loisel is rare (Bornschein et al. 2017). Subtropical salt marshes are associated with mangroves, distributed thinly in Brazil from the estuaries of the central-south coast of São Paulo to the north coast of Santa Catarina (Bornschein et al. 2017).
Currently, South American salt marshes are being invaded and dominated by tanner grass Urochloa arrecta (Hack. ex T. Durand and Schinz) Morrone and Zuloaga, which causes major problems for the region (Reinert et al. 2007). This species, when established, profoundly alters the communities in the environment (Casatti et al. 2009; Michelan et al. 2010; Carniatto et al. 2013). This poses a great threat to the conservation of endemic birds in southern Brazil, such as the marsh antwren Formicivora acutirostris (Bornschein, Reinert & Teixeira, 1995), which was first described in 1995 (Bornschein et al. 1995; Reinert et al. 2007). Environments completely invaded by this exotic grass are no longer occupied by the bird, which is why the impact is considered an area suppressor (Reinert et al. 2007). In contrast, marshes in California, in the United States, were invaded by a hybrid species of Spartina that benefited populations of the threatened Ridgway’s rail Rallus obsoletus Ridgway 1874 (taxonomy according to Maley and Brumfield [2013]). The hybrid increased the survival of this bird by providing refuge against predators during extreme tides that inundated native vegetation, particularly during winter, when native vegetation entered senescence (Overton et al. 2014). Hybrid plant management programs have reduced the survival rate of R. obsoletus and plans for its conservation suggest offering refuge against the high tides caused by rising sea levels (Overton et al. 2014).
In 2006–2022, salt marshes in southern Brazil were studied as part of a long-term project aiming to monitor and conserve F. acutirostris—a vulnerable (VU) species at risk of extinction in Brazil (Ordinance 148 of the Brazilian Ministério do Meio Ambiente [MMA], June 7, 2022). This bird is included in the Brazilian National Action Plan for the conservation of Atlantic Forest Birds, one of the specific objectives of which is to deter and control alien species in the bird’s natural habitat (Ordinance 208 of the Brazilian MMA and Instituto Chico Mendes de Conservação da Biodiversidade [ICMBio], March 3, 2018). In 2012 conservation efforts to protect F. acutirostris involved a challenging program that aimed to eradicate U. arrecta (Bornschein 2013), which impacted a local population of the mangrove rail Rallus longirostris Boddaert 1783. Rallus longirostris is generally restricted to mangroves (Vieira 2015) and is distributed across small portions of the Pacific coastal region, Central America, northern South America, and vast stretches of the Atlantic coast in South America (Maley et al. 2016). The species’ geographic distribution primarily occurs along the Atlantic coast of Brazil—a country in which its conservation status was officially considered to be of least concern (LC; ICMBio 2018), although it may be threatened with extinction (Vieira 2015). In this article, we report on the appearance and distributional expansion of R. longirostris in subtropical salt marshes invaded by U. arrecta and its disappearance after the plant was eradicated. We also discuss the possible causes of this event.
Methods
Study species
The target species was the mangrove rail Rallus longirostris, which is considered a separate species from the North American R. obsoletus and the clapper rail R. crepitans Gmelin 1789 (Maley and Brumfield 2013; Chesser et al. 2014).
Study areas and field time
We worked at the Ramsar Site Guaratuba in the Guaratuba Bay estuary in Guaratuba Municipality, which is on the southern coast of Paraná in southern Brazil. Specifically, we studied four areas: Jundiaquara Island (c. 25°52’25”S, 48°45’32”W; 11.30 ha), the confluence of the Claro and São João Rivers (“Continente”; c. 25°52’28 “S, 48°45’44”W; 8.47 ha), Folharada Island and its surroundings (c. 25°51’58”S, 48°43’23”W; 30.72 ha), and the Riozinho River mouth (“Riozinho”; c. 25° 52’00”S, 48°45’05”W; 3.98 ha; see Favretto et al. 2022). Folharada Island and its surroundings are located downstream, and the remaining areas are further upstream, in the São João river.
The study areas are estuarine marshes (Doody 2001: 65), tidal marshes (Reinert et al. 2007), or subtropical salt marshes (Bornschein et al. 2017). According to the criteria for the classification of Brazilian vegetation proposed by the RADAMBRASIL Project (Veloso et al. 1991), marshes are pioneer formations with fluviomarine influences. The following herbaceous species dominate: C. americanum (Amaryllidaceae), S. californicus, Fuirena robusta Kunth, Cladium mariscus (L.) Pohl (Cyperaceae), Acrostichum danaeifolium Langsd. and Fisch. (Pteridaceae), and Stephostachys mertensii (Roth) Zuloaga and Morrone (Poaceae) (Reinert et al. 2007). The herbaceous southern cattail Typha domingensis Pers. also occurs locally (Typhaceae; Fig. 1 A) together with trees such as Calophyllum brasiliense Cambess. (Clusiaceae), Annona glabra L. (Annonaceae), and Laguncularia racemosa (L.) C.F. Gaertn. (Combretaceae) (Reinert et al. 2007). The areas are characterized by mixed semi-diurnal tides (Lee and Chang 2019), with two high tides and two low tides of different amplitudes on all lunar days.
(A) Subtropical salt marsh dominated by the crinum lily Crinum americanum L. and the southern cattail Typha domingensis Pers. (Crinum-Typhetum), with mangroves of Laguncularia racemosa (L.) C.F. Gaertn in the background on the left of the image (Laguncularietum; Folharada Island). (B) A subtropical salt marsh invaded by the alien Urochloa arrecta (Hack. ex T. Durand and Schinz) Morrone and Zuloaga (Urochloetum; Patch 1, Riozinho). Guaratuba Bay, Guaratuba Municipality, Paraná, southern Brazil. Photograph: Marcos R. Bornschein
We characterized the vegetation (phytophysiognomy) according to one or two dominant plant species. We consider as becoming a phytophysiognomy the vegetation with at least three plant species, but two of them in dominance, with the living aerial parts of each covering at least 20% of the soil surface. For this evaluation, we defined more than 1,000 phytosociological description plots of 1 m2 (Braun-Blanquet 1979; see Favretto et al. 2022). We also estimated the soil coverage by the living aerial parts of the plants systematically, according to Bolòs et al. (1991). We refer to each phytophysiognomy using the suffix “etum”, according to Braun-Blanquet (1979).
The studies began in January 2006 on Jundiaquara Island and at Riozinho, in 2007 at Continente, in 2009 on Folharada Island, and in 2011 in the surroundings of Folharada Island. In all areas, we carried out the work until August 2022. Between January 2006 and May 2008, we worked in the areas daily from September to February and for 6–8 days per month for the rest of the year. From 2009 onward, we worked in the areas for 3–8 days per month, every month. We did not work in the areas for six straight months (March–August) in 2020 due to the COVID-19 pandemic. Fieldwork was carried out by 2–7 people (usually three) accessing the areas by boat. On each fieldwork day, we worked from dawn until 12 p.m. or 1 p.m., and for a further 2–3.5 h in the afternoon, before dusk.
This work occurred in concert with research focused on F. acutirostris (see Reinert et al. 2012; Bornschein et al. 2015), the wren-like rushbird Phleocryptes melanops (Vieillot 1817), and the many-colored rush tyrant Tachuris rubrigastra (Vieillot 1817) (see Favretto et al. 2022). Our quantitative sampling consisted of point counts of all species of birds seen or heard within a 50 m radius of the observer (Bibby et al. 1998; see also Bornschein et al. 2017), for 15 min at each point. We established two bird censuses, each with six-point counts in subtropical salt marshes. Six points were allocated to Jundiaquara Island and Continente and six to Folharada Island and its surroundings. The edge of each point was at least 45 m from the edge of the closest point. The census was conducted on consecutive days each month, with sessions starting at sunrise and interrupted only by rain. Rainy days or extremely low tides downstream prevented us from conducting many censuses or completing bird counts at all six points of each census. The census results were recorded as punctual abundance index (PAI) values, which were obtained by summing all recorded individuals per species and dividing the number by the number of points in each census (Uezu et al. 2005).
Local impact of Urochloa arrecta
In patches in the studied areas, the alien African grass Urochloa arrecta (or Brachiaria subquadrippara) was the dominant species (Urochloetum; Fig. 1B). In these patches, native species co-occurred with this alien species but at low frequencies, because U. arrecta had become a dominant species due to the accumulation of stolons that shade and crush native vegetation, killing native plants (Fig. 2B; Reinert et al. [2007]). This process replaces the normal salt marsh vegetative structure and impairs bird displacement in the vegetation understory by obstructing ground space with intermixed stems that prevent movement through the marsh. (Fig. 2 A; Reinert et al. [2007]). Urochloa arrecta also advances on the water as floating banks of vegetation that are sometimes ripped out by floods and transferred to other areas previously free of their presence (Reinert et al. 2007).
Comparison of the vertical structure of a subtropical salt marsh (A) dominated by the crinum lily Crinum americanum L. and the southern cattail Typha domingensis Pers. (Crinum-Typhetum) with a place (B) dominated by the alien Urochloa arrecta (Hack. ex T. Durand and Schinz) Morrone and Zuloaga (Urochloetum; Patch 1) and hosting the California bulrush Schoenoplectus californicus (C.A. Mey) Soják. In B, the edge of the vegetation was resulted from partial cutting, due to management intervention. Guaratuba Bay, Guaratuba Municipality, Paraná, southern Brazil. Photographs: Marcos R. Bornschein
Management of Urochloa arrecta
Urochloa arrecta was mechanically managed to ensure its complete local eradication, with permissions from the Instituto Ambiental do Paraná (357/11) and Instituto Água e Terra (12.20). The eradication was achieved by clear-cutting vegetation with brush cutters and stacking plant biomass without using herbicides (Bornschein 2013). The stacked biomass was contained with stakes to prevent movement by high tides (Fig. 3).
Management of Urochloa arrecta (Hack. ex T. Durand and Schinz) Morrone and Zuloaga that invaded the subtropical salt marsh in southern Brazil. Management consisted of clear-cutting the vegetation with brush cutters and piling up the biomass, which was stacked with bamboo supports to prevent it from being carried away by high tides (since the water usually almost reached the tops of the highest piles). The managed area was inspected to manually remove of sprouts of exotic grass up to six times, and the biomass piles were turned over regularly up to six times to ensure the death of sprouts. Photographs: Marcos R. Bornschein
Cut and stacked piles of biomass were mixed up to six times to isolate living material by pulling dead vegetation from the interior of the pile to the edge and removing and reburying rooted and sprouted fragments (Bornschein 2013). Urochloa arrecta does not form seed banks locally, which facilitated successful management techniques. As a fast-growing pioneer formation, with the sprouting of plants or germination of seeds, natural regeneration fully developed within about six months of management commencing. In 7 months, the landscape was completely changed, and in 10 months, the native vegetation covered the land again, free of alien species (Bornschein 2013). The resulting environment following the intervention was dominated by S. californicus. This is common in tidal marsh environments, especially along riverbanks (Bornschein 2001; Reinert et al. 2007). We delimited and measured polygons of areas invaded by U. arrecta and monitored them with the Google Earth Pro program (7.3.3.7786) for each year (2003–2006) for which images were available (Table 1).
Results
Records of Rallus longirostris and nesting points
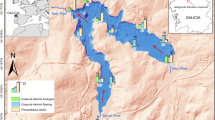
We recorded Rallus longirostris for the first time in the study region in 2007, one year after the beginning of our studies in Guaratuba Bay. We observed two individuals vocalizing in duet in a patch dominated by U. arrecta (Urochloetum) in Riozinho (Patch 1; Fig. 4). In 2010 this patch of Urochloetum covered 1.23 ha (Table 1). This bird was always observed in Patch 1, where it also nested, and in the surrounding salt marshes free of U. arrecta (Fig. 4 C). Two photographs on the WikiAves website (https://www.wikiaves.com.br/), one from 2007 and another from 2012 (WA280252 and WA832943)—document the presence of the bird in the surroundings of Patch 1.
The area studied in 2006–2022 in Guaratuba Bay, Guaratuba Municipality, Paraná, southern Brazil (polygons with yellow limits; A–B), B) Indications of patches dominated by the alien Urochloa arrecta (Hack. ex T. Durand and Schinz) Morrone and Zuloaga (at their 2010 size; white polygons) C) Maximum area with the occurrence of Rallus longirostris Boddaert, 1783 (red polygons). Map generated with ArcGIS Pro. Imagery source: Instituto Brasileiro de Geografia e Estatistica (IBGE), National Aeronautics and Space Administration (NASA), National Geospatial-Intelligence Agency (NGA), United States Geological Survey (USGS), Esri, HERE, Garmin, Foursquare, GeoTechnologies, Inc, Maxar, Ministry of Economy, Trade, and Industry (METI)/NASA
In 2010, we recorded R. longirostris at the Continente in a 0.10-ha patch of Urochloetum (Patch 4; Table 1; Fig. 4), three years after the beginning of the studies in this area. We observed three nests containing eggs in subsequent years on a mass of U. arrecta stolons. We also observed lone individuals or pairs of R. longirostris in surrounding salt marshes free of U. arrecta up to 400 m away from Patch 4 (Fig. 4 C).
In 2016, we recorded R. longirostris for the first time in a 0.57-ha patch of Urochloetum (Patch 2; Table 1; Figs. 4), 10 years after the beginning of our studies in this area. On Patch 3, with up to 0.23 ha of Urochloetum (Table 1; Fig. 4), we recorded no R. longirostris.
In 2012, we started managing U. arrecta in Patch 1 (see Table 1). In 2015, we continued to record R. longirostris in this patch (Table 1), and on one occasion, we observed R. longirostris feeding on Uca mordax on the mudflat, but chased away by management brush cutters. The bird was nesting on top of a biomass pile of managed vegetation (but the eggs were preyed upon). In 2016, Patch 1 of Urochloetum was practically eliminated, and since then, R. longirostris has not been recorded at this location. At the end of 2016, we started managing U. arrecta in Patch 4, practically eliminating this grass in 2017, after which we no longer recorded R. longirostris there (Table 1). During 2017 and 2018, we managed U. arrecta on Patch 2, but we were unable to eliminate it before financial resources expired. When management ended, the Urochloetum patch started to expand again (Table 1). Finally, we resumed management in 2020, eliminating U. arrecta in the same year, and recording our last observation of R. longirostris in the study region (Table 1).
During the censuses (N = 151; Table S1), we recorded R. longirostris occasionally in 2013–2015, in small numbers in subtropical salt marshes, but only in censuses conducted near patches of Urochloetum (Table 2). On Folharada Island and in the surrounding area, where U. arrecta was absent, we recorded no R. longirostris (Table 2; Fig. 4). In this area, we started our studies in 2009 and the censuses in 2011.
Use of the environment by Rallus longirostris and its relationship with other Rallidae
We observed R. longirostris occupying mudflats where the management had removed U. arrecta from patches of Urochloetum, and in native subtropical salt marsh vegetation. The subtropical salt marshes phytophysiognomies used by R. longirostris were the ones dominated by S. californicus (Schoenoplectetum), C. americanum and S. californicus (Crinum-Schoenoplectetum), C. americanum and F. robusta (Crinum-Fuirenetum), C. americanum and T. domingensis (Crinum-Typhetum), C. americanum and C. mariscus (Crinum-Cladietum), and, finally, by A. danaeifolium (Acrostichetum). Regarding these phytophysiognomies, we observed R. longirostris in the lower layer of the vegetation (Fig. 5 A), occupying the sediment between the plants and accumulated dead leaves, and in the intermediate layer, which contained a high density of live and dead leaves (Fig. 5 A). We did not observe R. longirostris in the upper layer of vegetation, where the plants were vertical and less dense than in the lower layers. In Urochloetum, we observed R. longirostris only on top of the plant mass. Because this bird does not occupy areas with dense stolons of U. arrecta (Fig. 5B), the Urochloetum surface functions as the lower layer of vegetation, which is quite open and exposed. It was in this layer that we observed the R. longirostris nests (see above). The remaining native vegetation in Urochloetum constitutes the upper layer, with no intermediate layer.
Schematic representation of subtropical salt marsh vegetation layers (A) and an area invaded and dominated by the alien Urochloa arrecta (Hack. ex T. Durand and Schinz) Morrone and Zuloaga (B), according to its use by the mangrove rail Rallus longirostris. Blue vertical bars indicate the lower layer, red vertical bars indicate the intermediate layer, and black vertical bars indicate the upper layer. Created with BioRender.com
We also observed blackish rail Pardirallus nigricans (Vieillot 1819), associated with the same phytophysiognomies and vegetation layers as R. longirostris, except on Urochloetum and mudflats (Fig. 6). We observed that coexisted only in Schoenoplectetum, with no evidence of aggression between them (Fig. 6). Pardirallus nigricans is the most abundant non-migratory species in the subtropical salt marshes (Table S2), and we saw this bird nesting in the intermediate layers of vegetation in all phytophysiognomies, except Schoenoplectetum and, obviously, Urochloetum. Other Rallidae are exceptional in subtropical salt marshes (Table 2) and we never observed them coexisting with R. longirostris.
Schematic representation of subtropical salt marshes invaded and not invaded by the alien Urochloa arrecta (Hack. ex T. Durand and Schinz) Morrone and Zuloaga (right third) relative to the occurrence and nidification of Rallus longirostris Boddaert, 1783 and Pardirallus nigricans (Vieillot, 1819). Created with BioRender.com
Discussion
This long-term study allowed us to verify (1) the appearance of a population of R. longirostris occupying and nesting in patches dominated by the exotic grass U. arrecta; (2) an increase in the population’s local distribution, occupying of other patches dominated by this plant and surrounding salt marshes free from the alien species; and (3) its local disappearance after the eradication of the plant, in salt marshes free from the alien plant. This constitutes a further case of a native species benefiting from an alien species invasion (Overton et al. 2014; Casazza et al. 2014) and provides evidence of a preference for an altered environment over a natural environment for nesting. This suggests that the subtropical salt marsh structurally impedes the nesting of R. longirostris (Hypothesis 1) or there is an ecological impediment to nesting in an environment not invaded by U. arrecta (Hypothesis 2).
Regarding Hypothesis 1, the most abundant resident bird at the study sites was P. nigricans (Table S2), which is similar in size to R. longirostris (Dunning 2008) and nests in subtropical salt marshes (Fig. 6). Locally, these two birds build nests as baskets constructed from fragments of native herbaceous plants of similar size, supported above the herbaceous vegetation (4 nests of R. longirostris; c. 150 nests of P. nigricans [unpublished data]). Thus, subtropical salt marshes are environments in which herbaceous plants support the construction of relatively large birds nests. However, it is known that due to rising tides, tidal marshes have far fewer nesting sites than foraging areas, and this characteristic shape the social organization of the environment (Post 1974). So, there is a high likelihood of disputes over reproductive sites between these birds in subtropical salt marshes (Hypothesis 2), with P. nigricans dominating R. longirostris. We suggest that R. longirostris could have occupied patches of Urochloetum as vacant nesting niches to avoid disputes with P. nigricans. There were other rail species in the subtropical salt marshes of the study region, but they occur in low numbers and only occasionally (Table 2); therefore, we did not expect that any ecological interaction with R. longirostris that influence its local ecology and preferences.
Rallus longirostris and P. nigricans do not seem to use the interiors of areas dominated by U. arrecta, possibly due to the high density of stolons (Fig. 2B) limiting their movements and access to food (MRB per. obs.). The non-use of this part of the very dense vegetation means that the invaded environment, regarding to its use by the rails, has only two layers—not three, as in non-invaded environments (Fig. 5). Hence, the invaded areas have vegetation with reduced structural complexity. Likewise, the lower and upper layers of areas invaded by U. arrecta are quite open (Fig. 5), which seems to be why P. nigricans do not occur or nest there. Rallus longirostris, in turn, seems to prefer less dense vegetation, since it breeds in the lower layer of invaded areas, and occurs in mangroves and even on mudflats.
The layers of native vegetation in the phytophysiognomies in which R. longirostris occurs comprise: a lower layer with low to moderate vegetation density, an intermediate layer with moderate to high vegetation density, and an upper layer with low vegetation density (Fig. 6). As a consequence of the rosette shape of this species, the phytophysiognomies where C. americanum is dominant are characterized by increments of leaves overlap in the intermediate layer of the vegetation, forming several “Ys” side by side. The top of each “Y” supports the dead leaves of other plant species, contributing to the high density of vegetation in the intermediate layer. The erect plants T. domingensis and C. mariscus develop leaves from their stems, which touch at mid-height of the vegetation and support dead leaves, contributing to increased vegetation density in the intermediate layer of phytophysiognomies where these species are the dominant plant species. In Schoenoplectetum, although S. californicus has smooth leaves without ramification, the three layers still have different vegetation densities because the leaves of intersect, forming multiple intersecting “Xs”. Additionally, the points of intersection of S. californicus leaves also accumulate dead leaves, contributing to increased density in the intermediate layer. However, this density tends to be much lower than that associated with other phytophysiognomies (Teixeira and Bornschein unplublished data).
Birds such as R. obsoletus generally prefer more complex environments for nest building (Rush et al. 2010). The apparent advantages of nesting in the lower layer in Urochloetum are the support for the nests and the buoyancy of the biomass under high-tide conditions, which could potentially reduce reproductive losses from flooding—an impact that is quite significant for wetland birds (Marshall and Reinert 1990; Shriver 2002; Greenberg et al. 2006; Reinert 2006; Norbdy et al. 2009). In the study region, high tides flood the nests of F. acutirostris (Reinert et al. 2012), the least bittern Ixobrychus exilis (Gmelin, 1789), P. nigricans, Phleocryptes melanops, the yellow-chinned spinetail Certhiaxis cinnamomeus (Gmelin, 1788), Tachuris rubrigastra, and the Brazilian tanager Ramphocelus bresilia (Linnaeus, 1766; MRB per. obs.).
The principle of equal opportunity (MacArthur 2002) predicts that the occupation of environments by species depends on the relationship between the resources in those habitats and the pressure to use them, meaning that individuals to a certain extent prefer less competitive environments, could be a factor in the choice of the environment by R. longirostris and P. nigricans and their consequent nesting in patches of U. arrecta by the former. The occupation of environments depends on competition (Cody 1985), and few species can occupy resource-poor habitats, such as patches of Urochloetum with impoverished flora and a simple vegetation structure, which leads to reduced competition (Cody 1985). Rallus longirostris could have benefited from the occupation of patches containing exotic plants. Although R. longirostris and P. nigricans coexist in vegetation free of exotic plants without apparent mutual aggression, even when they are side by side, the similarity between these species suggests that they compete silently without obvious aggression (MacArthur 2002).
Benefits were verified for the congeneric R. obsoletus following the invasion of a hybrid form of Spartina grass, which increased the survival rate of individuals by offering refuge against predators (Overton et al. 2014). Following the introduction of an exotic-plant eradication program, the bird population declined (McBroom 2013), as observed in the present study of R. longirostris. Conversely, biological invasions of clonal grass Phragmites australis (Cav.) Trin. ex Steud. in Canada had long-term negative impacts on birds, decreasing species richness and changing community compositions (Robichaud and Rooney 2017).
Rallus longirostris is not threatened with extinction either globally (BirdLife International 2016) or in Brazil (Ordinance 148 of the Brazilian MMA, June 7 2022), but it has been deemed VU in that country (Vieira 2015). Regionally, R. longirostris is considered VU in the state of Paraná (Decree 1.797/2018 of the State of Paraná, November 22, 2018), where the present research was carried out. The fact that R. longirostris has benefited from areas dominated by an exotic plant, allowing it to colonize a previously uncolonized environment (Vieira 2015), does not seem to justify interrupting or preventing the management of U. arrecta. It is a distinctly aggressive species (Kissman 1997; Thomaz et al. 2009) that reduces the functional diversity of native species due to the presence of allelopathic compounds and its high energy efficiency, resistance to drought periods, and high rates of germination, growth, regrowth, and regeneration (Freitas and Pivello 2005; Bianchini Jr. et al. 2010). The impacts of this grass on native macrophyte (Michelan et al. 2010) and fish (Casatti et al. 2009; Carniatto et al. 2013) communities have been reported, as well as the impact of habitat suppression on F. acutirostris (Reinert et al. 2007)—a species at risk of extinction in Brazil (Ordinance 148 of the Brazilian MMA, June 7, 2022). Moreover, the high cost of management and the difficulty of raising funds for this activity make it impossible to implement a program with the objective of eradicating some areas invaded by U. arrecta, but not all of them to sustain a population of R. longirostris. Permanent management of the Urochloetum to keep them with the same extension would make the program very expensive and unrealistic. The cost of restoring 1 ha dominated by the alien U. arrecta varies from 13,404 to 29,356 USD, depending particularly on the traveling distance by boat to access the areas requiring management (Teixeira and Bornschein unpublished data).
The use of subtropical salt marshes and areas dominated by exotic grasses by R. longirostris demonstrates ecological plasticity. With the advancing invasion of U. arrecta in estuaries, this new nesting niche of R. longirostris may increase its population and warrant reversion to its previously endangered status (Vieira 2015). Conversely, invasive plants can be ecological traps, attracting species but not sustaining them in the long term (Norbdy et al. 2009; Kloskowski 2012; Stinson and Pejchar 2018). Long-term monitoring of different estuaries is encouraged because it may reveal this or other population trends of R. longirostris, as confirmed by those presented here, in addition to allowing a deeper assessment of the impacts of U. arrecta invasions.
Data availability
The raw data in this article are included in the tables and figures.
Change history
09 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13157-023-01667-6
References
Battisti C (2016) Experiential key species for the nature-disconnected generation. Anim Conserv 31(5):627–644. https://doi.org/10.1080/08927936.2018.1505344
Battisti C, Fanelli G, Bertolino S, Luiselli L, Amori G, Gippoliti S (2018) Non-native invasive species as paradoxical ecosystem services in urban conservation education. Web Ecol 18:37–40. https://doi.org/10.5194/we-18-37-2018
Bellard C, Cassey P, Blackburn TM (2016) Alien species as a driver of recent extinctions. J R Soc 12(2):20150623. https://doi.org/10.1098/rsbl.2015.0623
Bianchini-Jr I, Cunha-Santino MB, Milan JA, Rodrigues CJ, Dias JHP (2010) Growth of Hydrilla verticillata (L.f.) Royle under controlled conditions. Hydrobiologia 644:301–312. https://doi.org/10.1007/s10750-010-0191-1
Bibby C, Jones M, Marsden S (1998) Expedition field techniques: bird surveys. Royal Geographical Society, London, United Kingdom
BirdLife International (2016) Rallus longirostris. The IUCN Red List of Threatened Species 2016: e.T62154828A95190148. Accessed December 27, 2021. https://doi.org/10.2305/IUCN.UK.2016-3.RLTS.T62154828A95190148.en
de Bolòs O, Cervi AC, Hatschbach G (1991) Estudios sobre la vegetación del estado de Paraná (Brasil Meridional). Collect Bot 20:79–182
Bornschein (2001) Formações pioneiras do litoral centro-sul do Paraná: identificação, quantificação de áreas e caracterização ornitofaunística. Dissertation, Universidade Federal do Paraná.
Bornschein MR (2013) Biologia da conservação do bicudinho-do-brejo Stymphalornis acutirostris (Aves, Thamnophilidae). Dissertation, Universidade Federal do Paraná
Bornschein MR, Pizo MA, Sobotka DD, Belmonte-Lopes R, Golec C, Machado-de-Souza T, Pie MR, Reinert BL (2015) Longevity records and signs of aging in Marsh Antwren Formicivora acutirostris (Thamnophilidae). Wilson j ornithol 127(1):98–102. https://doi.org/10.1676/14-074.1
Bornschein MR, Reinert BL, Machado-de-Souza T, Golec C, Whitney BM, Favretto MA (2017) Abundance, occurrence, and seasonality of the Subtropical Doradito (Pseudocolopteryx acutipennis) on the coast of Brazil. Wilson J Ornithol 129(1):199–206. https://doi.org/10.1676/1559-4491-129.1.199
Bornschein MR, Reinert BL, Teixeira DM (1995) Um novo Formicariidae do sul do Brasil (Aves, Passeriformes). Instituto Iguaçu de Pesquisa e Proteção Ambiental, Rio de Janeiro.
Braun-Blanquet J (1979) Fitosociologia: bases para el estúdio de las comunidades vegetales, 1 edn. st ed. H. Blume Ediciones, Madrid
Carniatto N, Thomaz SM, Cunha ER, Fugi R, Ota R (2013) Effects of an invasive alien Poaceae on aquatic macrophytes and fish communities in a neotropical reservoir. Biotropica 45(6):747–754. https://doi.org/10.1111/btp.12062
Casatti L, Ferreira CP, Carvalho FR (2009) Grass-dominated stream sites exhibit low fish species diversity and dominance by guppies: an assessment of two tropical pasture river basins. Hydrobiologia 632:273–283. https://doi.org/10.1007/s10750-009-9849-y
Casazza ML, Overton CT, Bui TVD, Hull JM, Albertson JD, Bloom VK, Bobzien S, McBroom J, Latta M, Olofson P, Rohmer TM, Schwarzbach S, Strong DR, Grijalva E, Wood JK, Skalos SM, Takekawa T (2014) Endangered species management and ecosystem restoration: finding the common ground. Ecol Soc 21:19. https://doi.org/10.5751/ES-08134-210119
Catling PM (2005) Effects of invasive alien plants on birds: some examples from North America. Biodivers 6(3):30–39. https://doi.org/10.1080/14888386.2005.9712772
Chesser RT, Banks RC, Cicero C, Dunn JL, Kratter AW, Lovette IJ, Navarro-Sigueza AG, Rasmussen PC, Remsem-Jr JV, Rising JD, Stotz DF, Winker K (2014) Fifty-fifth supplement to the american ornithologists’ Union check-list of North American Birds. Auk 131(4):CSi–CSxv. https://doi.org/10.1642/AUK-14-124.1
Cody MS (1985) Habitat selection in birds. Academic Press Inc., Orlando
Cuassolo F, Balseiro E, Modenutti B (2012) Alien vs. native plants in a Patagonian wetland: elemental ratios and ecosystem stoichiometric impacts. Biol Invasions 14:179–189. https://doi.org/10.1007/s10530-011-9995-9
Doody JP (2001) Coastal conservation and management: an ecological perspective. Kluwer Academic Publishers, Boston
Dunning JB (2008) Handbook of avian body masses, 2nd edn. CRC Press, Boca Raton
Favretto MA, Machado-de-Souza T, Golec C, Reinert BL, Bornschein MR (2022) Habitat selection in many-colored rush tyrant (Tachuris rubrigastra) and wren-like Rushbird (Phleocryptes melanops) in the subtropical salt marshes of Brazil. Stud Neotrop Fauna Environ. https://doi.org/10.1080/01650521.2022.2101351 .
Freitas GK, Pivello V (2005) A ameaça das gramíneas exóticas à biodiversidade. In: Freitas GK, Pivello VR (eds) O o cerrado Pé-de-Gigante: ecologia e conservação - parque Estadual de Vassununga. SMA, São Paulo, pp 234–246
Gedan K, Altieri A, Bertness MD (2011) Uncertain future of New England salt marshes. Mar Ecol Prog Ser 434:229–237. https://doi.org/10.3354/meps09084
Goodenough A (2010) Are the ecological impacts of alien species misrepresented? A review of the “native good, alien bad” philosophy. Community Ecol 11(1):13–21. https://doi.org/10.1556/ComEc.11.2010.1.3
Greenberg R, Elphick C, Nordby JC, Gjerdrum C, Spautz H, Shriver G, Schmeling B, Olsen B, Marra P, Nur N, Winter M (2006) Flooding and predation: trade-offs in the nesting ecology of tidal-marsh sparrows. Stud in Avian Biol 32:96–109
Hobbs RJ, Higgs E, Harris JA (2009) Novel ecosystems: implications for conservation and restoration. Trends Ecol Evol 34(11):599–605. https://doi.org/10.1016/j.tree.2009.05.012
ICMBio (2018) Livro vermelho da fauna brasileira ameaçada de extinção, vol 1. ICMBio & MMA, Brasília
Kissman KG (1997) Plantas infestantes e nocivas, vol 3. BASF, São Paulo
Lee SH, Chang YS (2019) Classification of the global tidal types based on auto-correlation analysis. Ocean Sci 52(2):279–286. https://doi.org/10.1007/s12601-019-0009-7
Kloskowski J (2012) Fish stocking creates an ecological trap for an avian predator via effects on prey availability. Oikos 121(10):1567–1576. https://doi.org/10.1111/j.1600-0706.2011.19942.x
Lees AC, Bell DJ (2008) A conservation paradox for the 21st century: the european wild rabbit Oryctolagus cuniculus, an invasive alien and an endangered native species. Mammal rev 38(4):304–320. https://doi.org/10.1111/j.1365-2907.2008.00116.x
Levin LA, Neira C, Grosholz ED (2006) Invasive cordgrass modifies wetland trophic function. Ecology 87(2):419–432. https://doi.org/10.1890/04-1752
MacArthur RH (2002) Geographical ecology: patterns in the distribution of species. Princeton University Press, Princeton
McBroom J (2013) California Clapper Rail surveys for the San Francisco estuary invasive Spartina Project 2013. State Coastal Conservancy, Oakland
Maley JM, Brumfield RT(2013) Mitochondrial and next-generation sequence data used to infer phylogenetic relationships and species limits in the Clapper/King Rail complex. Condor115(2):316–329. https://doi.org/10.1525/cond.2013.110138
Marshall RM, Reinert SE (1990) Breeding ecology of seaside sparrows in a Massachusetts salt marsh. Wilson Bull 102:501–513
Meyerson LA, Mooney HA (2007) Invasive alien species in an era of globalization. Front Ecol Environ 5(4):199–208. https://doi.org/10.1890/1540-9295(2007)5[199:IASIAE]2.0.CO;2
Michelan TS, Thomaz SM, Mormul RP, Carvalho P (2010) Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Freshw Biol 55:1315–1326. https://doi.org/10.1111/j.1365-2427.2009.02355.x
Norbdy JC, Cohen NA, Beissinger SR (2009) Effects of a habitat-altering invader on nesting sparrows: an ecological trap? Biol Invasions 11:565–575. https://doi.org/10.1007/s10530-008-9271-9
Overton CT, Casazza ML, Takekawa JY, Strong DR, Holyoak M (2014) Tidal and seasonal effects on survival rates of the endangered California Clapper Rail: does invasive Spartina facilitate greater survival in a dynamic environment? Biol Invasions 16:1897–1914. https://doi.org/10.1007/s10530-013-0634-5
Post W (1974) Functional analysis of space-related behavior in the seaside sparrow. Ecology 55:564–575. https://doi.org/10.2307/1935147
Reinert BL, Belmonte-Lopes R, Bornschein MR, Sobotka DD, Corrêa L, Pie MR, Pizo MA (2012) Nest and eggs of the Marsh Antwren (Stymphalornis acutirostris): the only marsh-dwelling thamnophilid. Wilson j ornithol 124(2):286–291. https://doi.org/10.1676/11-099.1
Reinert BL, Bornschein MR, Firkowski C (2007) Distribuição, tamanho populacional, hábitat e conservação do bicudinho-do-brejo Stymphalornis acutirostris Bornschein, Reinert e Teixeira, 1995 (Thamnophilidae). Rev Bras Ornitol 15:493–519
Reinert SE(2006) Avian nesting response to tidal-marsh flooding: literature review and a case for adaptation in the red-winged blackbird. In: Greenberg R., Maldonado JE, Droege S, McDonald MV (eds). Terrestrial vertebrates of tidal marshes: Evolution, ecology, and conservation. Stud Avian Biol 32:77–95
Robichaud CD, Rooney RC (2017) Long-term effects of a Phragmites australis invasion on birds in a Lake Erie coastal marsh. J Great Lakes Res 43(3):141–149. https://doi.org/10.1016/j.jglr.2017.03.018
Rush AS, Woodrey MS, Cooper RJ (2010) Variation in the nesting habitats of Clapper Rails in tidal marshes of the northern Gulf of Mexico. Condor 112(2):356–362. https://doi.org/10.1525/cond.2010.090078
Schlaepfer MA, Runge MC, Sherman PW (2002) Ecological and evolutionary traps. Trends Ecol Evol 17:474–480. https://doi.org/10.1016/S0169-5347(02)02580-6
Shriver WG(2002) Conservation ecology of salt marsh birds in New England. Dissertation, State University of New York
Simberloff D (2007) Given the stakes, our modus operandi in dealing with invasive species should be “guilty until proven innocent”. Conserv Magazine 8:1819
Stinson LT, Pejchar L (2018) The effects of introduced plants on songbird reproductive success. Biol Invasions 20:1403–1416. https://doi.org/10.1007/s10530-017-1633-8
Thomaz SM, Carvalho P, Mormul RP, Ferreira FA, Silveira MJ, Michelan TS (2009) Temporal trends and effects of diversity on occurrence of exotic macrophytes in a large reservoir. Acta Ecol 35(5):614–620. https://doi.org/10.1016/j.actao.2009.05.008
Uezu A, Metzger JP, Vielliard JME (2005) Effects of structural and functional connectivity and patch size on the abundance of seven Atlantic Forest bird species. Biol Conserv 123:507–519. https://doi.org/10.1016/j.biocon.2005.01.001
Veloso HP, Rangel Filho ALR, Lima JC (1991) Classificação da vegetação brasileira, adaptada a um sistema universal. IBGE, Brasil
Vieira BP (2015) Population trends and conservation of the Mangrove Rail. Rev Bras Ornitol 23(3):327–335. https://doi.org/10.1007/BF03544301
Vitousek PM, Antonio CMD, Loope LL, Rejmánek M, Westbrooks R (1997) Introduced species: a significant componente of human-caused global change. NZJ Ecol 21:1–16
Watson EB, Byrne R (2009) Abundance and diversity of tidal marsh plants along the salinity gradient of the San Francisco Estuary: implications for global change ecology. Plant Ecol 205:113–128. https://doi.org/10.1007/s11258-009-9602-7
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Crit Rev Plant Sci 23:431–452. https://doi.org/10.1080/07352680490514673
Acknowledgements
We obtained management permissions from the Instituto Ambiental do Paraná (357/11) and Instituto Água e Terra (12.20). This study was supported by Fundação Grupo Boticário de Proteção à Natureza (FGBPN; projects 0682/20052; 0740/20071; 0908_20112; BL0001_20111, 0004_2012, and 1110_20172), Fundo Brasileiro para a Biodiversidade (FUNBIO), and 1ª Vara Federal de Paranaguá (process 50005063420184047008). Most of these projects were developed by Mater Natura—Instituto de Estudos Ambientais, with special financial management support from Helena Zarantonielli. Ricardo Belmonte-Lopes, Daiane D. Sobotka, Cláudia Golec, Leandro Corrêa, Mario Arthur Favretto, Tiago Machado-de-Souza, and Ailton Degues helped with the fieldwork. We thank the two anonymous reviewers who carefully reviewed our manuscript and made valuable suggestions for improving the quality of the study.
Funding
This study was supported by Fundação Grupo Boticário de Proteção à Natureza (FGBPN; projects 0682/20052; 0740/20071; 0908_20112; BL0001_20111, 0004_2012, and 1110_20172), Fundo Brasileiro para a Biodiversidade (FUNBIO), and 1ª Vara Federal de Paranaguá (process 50005063420184047008).
Author information
Authors and Affiliations
Contributions
Conceptualization: Marcos R. Bornschein. Field procedures: Marcos R. Bornschein, Larissa Teixeira, Bruno de Morais Guerra, Bianca L. Melchiori, Bianca L. Reinert, Giovanna Sandretti-Silva. Funding acquisition: Bianca L. Reinert. Field administration: Marcos R. Bornschein, Larissa Teixeira, Bianca L. Reinert and Giovanna Sandretti-Silva. Writing: Marcos R. Bornschein, Larissa Teixeira, Bruno de Morais Guerra, Bianca L. Melchiori and Giovanna Sandretti-Silva. Preparing figures and/or tables: Marcos R. Bornschein, Larissa Teixeira and Bruno de Morais Guerra.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant competing interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
†Bianca L. Reinert In memoriam.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bornschein, M.R., Teixeira, L., de Morais Guerra, B. et al. Appearance of a population of the mangrove rail Rallus longirostris (Rallidae) in salt marshes invaded by the exotic tanner grass Urochloa arrecta (Poaceae) and its disappearance after plant management. Wetlands 42, 124 (2022). https://doi.org/10.1007/s13157-022-01642-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-022-01642-7