Abstract
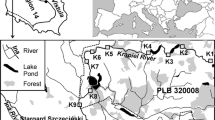
The factors influencing molluscs distribution in a small steppe stream were investigated to assess the impact of beavers on their diversity and abundance. Twenty stations—in unaffected streams, beaver ponds at different stages of development, and anthropogenic ponds—were sampled in May 2019 and June 2021. In each pond, a nearshore area of 1 m2 was completely surveyed using a dip net. Twenty-one mollusc species were found. Old and drained beaver ponds were characterized by significantly higher species richness. The damming of the river, by both humans and beavers, initially resulted in decreased species diversity, then followed by increased species richness due to increased habitat heterogeneity across the valley. Maximum gamma biodiversity was observed when the river habitats included all types of beaver ponds. When ponds were abandoned, a short-term increase in mollusc biodiversity and abundance was observed. Afterward, channel processes returned the habitats to a lotic state, habitat heterogeneity began to decrease, and mollusc community evenness dropped dramatically. To conserve the mollusc biodiversity of the stream valley, it is necessary for beavers to permanently renew some ponds.
Aннoтaция
B paбoтe aнaлизиpyютcя фaктopы, влияющиe нa pacпpeдeлeниe мoллюcкoв в дoлинe мaлoй cтeпнoй peки, для oцeнки влияния бoбpoв нa иx paзнooбpaзиe и чиcлeннocть. Были пpoaнaлизиpoвaны пpoбы, coбpaнныe в мae 2019 г. и в июнe 2021 г. нa двaдцaти yчacткax peки – в нeзaпpyжeннoм вoдoтoкe, бoбpoвыx пpyдax paзныx cтaдий paзвития и в aнтpoпoгeнныx пpyдax. B пpибpeжнoй чacти кaждoгo вoдoeмa был пoлнocтью oблoвлeн 1 м2 c пoмoщью гидpoбиoлoгичecкoгo caчкa. Oбнapyжeн 21 вид мoллюcкoв. Для cтapыx и cпyщeнныx бoбpoвыx пpyдoв xapaктepнo cтaтиcтичecки дocтoвepнoe yвeличeниe видoвoгo бoгaтcтвa. Изнaчaльнoe зaпpyживaниe peки, кaк чeлoвeкoм, тaк и бoбpaми, пpивoдит к cнижeнию биopaзнooбpaзия, зa кoтopым cлeдyeт eгo yвeличeниe – блaгoдapя пocтeпeннoмy yвeличeнию paзнooбpaзия мecтooбитaний в пpeдeлax вceй дoлины. Maкcимaльнoe гaммa-paзнooбpaзиe мoллюcкoв мoжeт нaблюдaтьcя, кoгдa в дoлинe peки вcтpeчaютcя вce типы пpyдoв. Кoгдa бoбpoвыe пpyды cтaнoвятcя бpoшeнными, этo пpивoдит к кpaткoвpeмeннoмy yвeличeнию paзнooбpaзия и чиcлeннocти мoллюcкoв. Bпocлeдcтвии, pycлoвыe пpoцeccы вoзвpaщaют тaкиe пpyды к иcxoднoмy пpoтoчнoмy cocтoянию, paзнooбpaзиe мecтooбитaний cнижaeтcя, и выpoвнeннocть cooбщecтв мoллюcкoв peзкo пaдaeт. Для coxpaнeния paзнooбpaзия мoллюcкoв мaлыx вoдoтoкoв, нeoбxoдимo чтoбы бoбpы oбнoвляли чacть cвoиx пpyдoв.






Similar content being viewed by others
Data Availability Statement
Data available from the Zenodo digital repository https://doi.org/10.5281/zenodo.6908506.
References
Anderson NL, Paszkowski CA, Hood GA (2015) Linking aquatic and terrestrial environments: can beaver canals serve as movement corridors for pond-breeding amphibians? Animal Conservation 18(3):287–294. https://doi.org/10.1111/acv.12170
Andreeva SI, Andreev NI, Vinarskii MV (2010) Identifier of freshwater gastropods (Mollusca: Gastropoda) of Western Siberia. Omsk [In Russian]
Andreyev NI, Krasnogorova AN, Andreeva SI (2010) Fauna of bivalve molluscs of the family Sphaeriidae in water bodies of the forest-steppe zone of Western Siberia and the Urals. Omsk Scientific Bulletin 1(94):243–246 [In Russian]
Aznar J-C, Desrochers A (2008) Building for the future: abandoned beaver ponds promote bird diversity. Écoscience 15(2):250–257. https://doi.org/10.2980/15-2-3107
Bashinskiy I (2014) Impact assessment of European beaver reintroduction on amphibians of small rivers. Russian Journal of Biological Invasions 5:134–145. https://doi.org/10.1134/S2075111714030035
Bashinskiy I (2021) Beaver impact on water coverage of forest-steppe territories (Penza Region, European Russia). Nature Conservation Research 6(1):88–97. https://doi.org/10.24189/ncr.2021.016
Bashinskiy I, Osipov V (2018) Distribution and dynamic of Castor fiber (Castoridae, Mammalia) population in forest-steppe rivers: a case of the State Nature Reserve Privolzhskaya Lesostep’, Penza region, European Russia. Nature Conservation Research 3:110–115. https://doi.org/10.24189/ncr.2018.068
Bashinskiy I, Senkevich V, Stoyko T, Katsman E, Korkina S, Osipov V (2019) Forest-steppe oxbows in limnophase — abiotic features and biodiversity. Limnologica 74:14–22. https://doi.org/10.1016/j.limno.2018.10.005
Bashinskiy I, Stoyko T, Senkevich V, Svinin A, Katsman E, Osipov V (2020) Structure and dynamics of mollusk communities of small oxbow lakes and the determining factors (the Khoper River Valley, Penza Oblast). Contemporary Problems of Ecology 13(6):631–642. https://doi.org/10.1134/S1995425520060037
Berg K, Ockelmann KW (1959) The respiration of freshwater snails. The Journal of Experimental Biology 36(4):690–708. https://doi.org/10.1242/jeb.36.4.690
Blöschl G, Hall J, Viglione A, Perdigão R, Parajka J, Merz B, …, Živković N (2019) Changing climate both increases and decreases European river floods. Nature 573: 108–111. https://doi.org/10.1038/s41586-019-1495-6
Brazier RE, Puttock A, Graham HA, Auster RE, Davies KH, Brown C (2020) Beaver: nature’s ecosystem engineers. Wiley Interdisciplinary Reviews: Water 8:1–29. https://doi.org/10.1002/wat2.1494
Brönmark C (1985) Freshwater snail diversity: effects of pond area, habitat heterogeneity and isolation. Oecologia 67(1):127–131
Bush BM, Stenert C, Maltchik L, Batzer DP (2019) Beaver-created successional gradients increase β-diversity of invertebrates by turnover in stream-wetland complexes. Freshwater Biology 64:1265–1274. https://doi.org/10.1111/fwb.13302
Bylak A, Kukuła K (2018) Living with an engineer: fish metacommunities in dynamic patchy environments. Marine and Freshwater Research 69:883–893. https://doi.org/10.1071/MF17255
Bylak A, Kukuła K (2022) Impact of fine-grained sediment on mountain stream macroinvertebrate communities: forestry activities and beaver-induced sediment management. Science of the Total Environment 832:155079. https://doi.org/10.1016/j.scitotenv.2022.155079
Bylak A, Kukuła K, Mitka J (2014) Beaver impact on stream fish life histories: the role of landscape and local attributes. Canadian Journal of Fisheries and Aquatic Sciences 71:1603–1615. https://doi.org/10.1139/cjfas-2014-0105
Bylak A, Szmuc J, Kukuła E, Kukuła K (2020) Potential use of beaver Castor fiber L., 1758 dams by the thick-shelled river mussel Unio crassus Philipsson, 1788. Molluscan Research 40:44–51. https://doi.org/10.1080/13235818.2019.1664371
Chen LY, Heath AG, Neves RJ (2001) Comparison of oxygen consumption in freshwater mussels (Unionidae) from different habitats during declining dissolved oxygen concentration. Hydrobiologia 450:209–214. https://doi.org/10.1023/A:1017501128572
Chertoprud MV, Udalov AA (1996) Ecological groups of freshwater gastropods in the central part of European Russia. The influence of the type of water body and substrate. Zoologicheskiy Zhurnal 75(5):664–676 [In Russian]
Chibilev AA (2002) Steppe and forest-steppe. In: Shahgedanova M (ed) The physical geography of Northern Eurasia. Oxford University Press
Czerniawski R, Sługocki Ł (2018) A comparison of the effect of beaver and human-made impoundments on stream zooplankton. Ecohydrology 11:e1963. https://doi.org/10.1002/eco.1963
Dalbeck L, Weinberg K (2009) Artificial ponds: a substitute for natural Beaver ponds in a Central European Highland (Eifel, Germany)? Hydrobiologia 630:49–62
Dalbeck L, Hachtel M, Campbell-Palmer R (2020) A review of the influence of beaver Castor fiber on amphibian assemblages in the floodplains of European temperate streams and rivers. Herpetological Journal 30:134–145. https://doi.org/10.33256/hj30.3.134145
Dgebuadze YY, Bashinskiy IV, Osipov VV (2021) The influence of Eurasian beaver Castor fiber activity on fish assemblages in small steppe rivers in Russia. Environmental Biology of Fishes 104(6):689–700. https://doi.org/10.1007/s10641-021-01103-w
Dgebuadze YY, Zavyalov NA, Petrosyan VG (eds.) (2012) European Beaver (Castor fiber L.) as a Key Species of a Small River Ecosystem (Prioksko-Terrasnyi Nature Biosphere Reserve). KMK Scientific Press, Moscow. [in Russian, with English summary]
Dillon RT (2000) The Ecology of Freshwater Mollusks. Cambridge University Press
Dmitrieva VA (2020) Modern changes in the water regime and morphometry of the rivers ш the Upper Don basin. Izvestia RAN Seriya Geographicheskaya 1:103–113. https://doi.org/10.31857/S2587556620010070 [InRussian]
Dolgin VN, Sviridenko BF (2011) Freshwater mollusks of the basins of the Pur and the Taz rivers (West Siberia). Tomsk State Pedagogical University Bulletin 110(8):89–92
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for flexible asymmetrical approach. Ecological Monographs 67:345–366
Ecke F, Levanoni O, Audet J, Carlson P, Eklöf K, Hartman G, …, Futter M (2017) Meta-analysis of environmental effects of beaver in relation to artificial dams. Environmental Research Letters 12: 113002. https://doi.org/10.1088/1748-9326/aa8979
France RL (1997) The importance of beaver lodges in structuring littoral communities in boreal headwater lakes. Canadian Journal of Zoology 75:1009–1013
Frolova NL, Nesterenko DP, Shenberg NV (2010) Intra-annual flow regime of rivers in Russia. Vestnik MGU Seriya Geograficheskaya 6:8–16 [In Russian])
Gashkina NA (2011) Zonal features of the distribution of biogenic elements and organic matter in small lakes. Water Resources 38(3):352–371
Gibson PP, Olden JD, O’Neill MW (2014) Beaver dams shift desert fish assemblages toward dominance by non-native species (Verde River, Arizona, USA). Ecology of Freshwater Fish 24(3):355–372. https://doi.org/10.1111/eff.12150
Gibson PP, Olden JD (2014) Ecology, management, and conservation implications of North American beaver (Castor canadensis) in dryland streams. Aquatic Conservation: Marine and Freshwater Ecosystems 24(3)391–409. https://doi.org/10.1002/aqc.2432
Gorczyca E, Krzemień K, Sobucki M, Jarzyna K (2018) Can beaver impact promote river renaturalization? The example of the Raba River, southern Poland. Science of the Total Environment 615:1048–1060. https://doi.org/10.1016/j.scitotenv.2017.09.245
Grigorev VY, Djamalov RG, Frolova NL (2017) River flow of the Oka and Don basins - its dynamics and causes of changes, Voprosy geografii. Sb. 145. Hydrological Changes. 194–205. [In Russian]
Grigoriev VY, Frolova NL (2018) Terrestrial water storage change of European Russia and its impact on water balance. Geography, Environment, Sustainability 11:38–50. https://doi.org/10.24057/2071-9388-2018-11-1-38-50
Halley DJ, Saveljev AP, Rosell F (2021) Population and distribution of beavers Castor fiber and Castor canadensis in Eurasia. Mammal Review 51:1–24. https://doi.org/10.1111/mam.12216
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1):9
Hood GA, Larson DG (2014) Beaver-created habitat heterogeneity influences aquatic invertebrate assemblages in boreal Canada. Wetlands 34(1):19–29. https://doi.org/10.1007/s13157-013-0476-z
Izmailova AV, Korneenkova NY (2020) Lake area percentage in russian federation territory and its governing factors. Water Resources 47(1):13–21. https://doi.org/10.1134/S009780782001008X
Johnson‐Bice SM, Gable TD, Windels SK, Host GE (2021) Relics of beavers past: time and population density drive scale‐dependent patterns of ecosystem engineering. Ecography (Cop.): e05814. https://doi.org/10.1111/ecog.05814
Jurkiewicz-Karnkowska E (2008) Aquatic mollusk communities in riparian sites of different size, hydrological connectivity and succession stage. Polish Journal of Ecology 56(1):99–118
Kappes H, Haase P (2012) Slow, but steady: dispersal of freshwater molluscs. Aquatic Sciences 74(1):1–14
Kemp PS, Worthington TA, Langford TEL, Tree ARJ, Gaywood MJ (2012) Qualitative and quantitative effects of reintroduced beavers on stream fish. Fish Fish 13:158–181. https://doi.org/10.1111/j.1467-2979.2011.00421.x
Khokhutkin IM, Vinarski MV, Grebennikov ME (2009) Mollusks of the Urals and the adjacent areas The family Lymnaeidae (Gastropoda, Pulmonata, Lymnaeiformes). P. 1. Goshchitskiy Publishers, Ekaterinburg. [In Russian]
Kijashko PV, Soldatenko EV, Vinarski MV (2016) Class Gastropoda Cuvier, 1797, Guide to zooplankton and zoobenthos of freshwaters European Russia. V. 2. Zoobenthos. KMK Scientific Press, Moscow-Saint-Petersburg. [In Russian]
Kirikov SV (1966) Game animals, natural environment and humans. Moscow, Nauka. [In Russian]
Kivinen S, Nummi P, Kumpula T (2020) Beaver-induced spatiotemporal patch dynamics affect landscape-level environmental heterogeneity. Environmental Research Letters 15:094065. https://doi.org/10.1088/1748-9326/ab9924
Koleszár G, Nagy Z, Peeters E, Borics G, Várbíró G, Birk S, Szabó S (2021) The role of epiphytic algae and grazing snails in stable states of submerged and of free-floating plants. Ecosystems. https://doi.org/10.1007/s10021-021-00721-w
Kornyushin AV (1996) Bivalve molluscs of the superfamily Pisidioidea of Palaearctica (fauna, systematics, phylogeny). Kiev: Institute of Zoology, National Academy of Sciences of Ukraine. [In Russian]
Kownacki A (1971) Taxocens of Chironomidae in streams of the Polish Hight Tatra, Mts. Acta Hydrobiologica 13(2):439–463
Krylov AV, Bobrov AA (eds.) (2007) Succession Changes in the Small River Ecosystem. KMK Scientific Press, Moscow. [in Russian, with English summary]
Kuznetsov MS, Kashtanov AN (2011) Distribution of soil erosion (map and explanatory note). In: National Atlas of Soils of the Russian Federation. Moscow, Astrel. [In Russian]
Law A, Mclean F, Willby NJ (2016) Habitat engineering by beaver benefits aquatic biodiversity and ecosystem processes in agricultural streams. Freshwater Biology 61:486–499. https://doi.org/10.1111/fwb.12721
Law A, Levanoni O, Foster G, Ecke F, Willby NJ (2019) Are beavers a solution to the freshwater biodiversity crisis? Diversity and Distributions 25:1763–1772. https://doi.org/10.1111/ddi.12978
Legendre P, Legendre L (1998) Numerical ecology, 2nd English ed. Elsevier
Little AM, Guntenspergen GR, Allen TFH (2012) Wetland vegetation dynamics in response to beaver (Castor canadensis) activity at multiple scales. Écoscience 19:246–257. https://doi.org/10.2980/19-3-3498
Lorencová E, Horsák M (2019) Environmental drivers of mollusc assemblage diversity in a system of lowland lentic habitats. Hydrobiologia 836(1):49–64
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press
Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. Annals of Mathematical Statistics 18:50–60
Margolis BE, Raesly RL, Shumway DL (2001) The effects of beaver-created wetlands on the benthic macroinvertebrate assem- blages of two Appalachian streams. Wetlands 21:554–563. https://doi.org/10.1672/0277-5212(2001)021[0554:TEOBCW]2.0.CO;2
Mikulka O, Homolka M, Drimaj J, Kamler J (2020) European beaver (Castor fiber) in open agricultural landscapes: crop grazing and the potential for economic damage. European Journal of Wildlife Research 66:101. https://doi.org/10.1007/s10344-020-01442-6
MolluscaBase eds. (2022) MolluscaBase. Accessed at https://www.molluscabase.org on 2022–02–06. https://doi.org/10.14284/448
Monakov A (1998) Diet of freshwater invertebrates. A.N. Severtsov Institute of Ecology and Evolution, Moscow [In Russian]
Mordukhay-Boltovskiy FD (ed) (1975) Methods to study biogeocenoses of inner water bodies. Nauka, Moscow [In Russian]
Myers JL, Well A, Lorch RF (2010) Research design and statistical analysis. Routledge/Taylor & Francis Group
Naiman RJ, Johnston CA, Kelley JC (1988) Alteration of North American streams by beaver. Bioscience 38(11):753–762. https://doi.org/10.2307/1310784
Nummi P (1989) Simulated Effects of the beaver on vegetation, invertebrates and ducks. Annales Zoologici Fennici 26(1):43–52
Nummi P, Holopainen S (2014) Whole-community facilitation by beaver: ecosystem engineer increases waterbird diversity. Aquatic Conservation: Marine and Freshwater Ecosystems 24:623–633. https://doi.org/10.1002/aqc.2437
Nummi P, Kuuluvainen T (2013) Forest disturbance by an ecosystem engineer: Beaver in boreal forest landscapes. Boreal Environment Research 18:13–24. https://doi.org/10.2514/6.2010-1764
Nummi P, Liao W, van der Schoor J, Loehr J (2021) Beaver creates early successional hotspots for water beetles. Biodiversity and Conservation. https://doi.org/10.1007/s10531-021-02213-8
Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, ..., Kassem KR (2010) Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience 51: 933–938. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
Orazi V, Hagge J, Gossner MM, Müller J, Heurich M (2022) A biodiversity boost from the eurasian beaver (Castor fiber) in Germany’s Oldest National Park. Frontiers in Ecology and Evolution 10. https://doi.org/10.3389/fevo.2022.873307
Paliy VF (1961) On quantitative indicators in the manipulation of fauna materials. Zoologicheskiy Zhurnal 60(1):3–12
Pollock MM, Naiman RJ, Erickson HE, Johnston CA, Pastor J, Pinay G (1995) Beaver as engineers: influences on biotic and abiotic characteristics of drainage basins. Linking species and ecosystems. Springer, Boston, 117–126. https://doi.org/10.1007/978-1-4615-1773-3_12
Prytkova MY (1982) Geographical patterns of sediment accumulation in small reservoirs. Summary DSc (Dr. of Geogr.) thesis, Leningrad. [In Russian]
Romashov BV, Romashov VA, Semenov VA, Filimonova LV (2005) Opisthorchiasis in the Upper Don Basin (Voronezh Region). Voronezh State University. [In Russian]
Rurek M (2021) Characteristics of Beaver ponds and landforms induced by beaver activity, S Part of the Tuchola Pinewoods, Poland. Water 13:3641. https://doi.org/10.3390/w13243641
Schlosser IJ, Kallemeyn LW (2000) Spatial variation in fish assemblages across a beaver-influenced successional landscape. Ecology 81(5):1371–1382. https://doi.org/10.1890/0012-9658(2000)081[1371:SVIFAA]2.0.CO;2
Shitikov VK, Rosenberg GS, Zinchenko TD (2003) Quantitative hydroecology: system identification methods, Togliatti: IEVB RAS. [In Russian]
Skal’skaya IA (2016) Peculiarities of the zooperiphyton succession in beaver ponds of a small river. Bulletin of the Russian Academy of Sciences 43:1365–1369. https://doi.org/10.1134/S1062359016100174
Spyra A (2018) Distribution patterns and habitat requirements of freshwater snails in man-made ponds. Annales Zoologici Fennici 55(1–3):1–14
Stadnichenko AP (1990) Physidae, Bulinidae and Planorbidae. Fauna of Ukaraine. T. 29. Mollusks. V. 4. Kiev: Naukova Dumka. [In Russian]
Stadnichenko AP (2004) Lymnaeidae and Acroloxidae of Ukraine. Kiev: Center of Educational Literature. [In Russian]
Starobogatov YI, Prozorova LA, Bogatov VV, Saenko EM (2004) Molluscs. In: Tsalolikhin SYa (eds) Key to freshwater invertebrates of Russia and adjacent lands. Molluscs, polychaetes, nemerteans, 6. Nauka, St. Petersburg, 9–491. [In Russian]
StatSoft, Inc (2004) STATISTICA (data analysis software system), version 7. www. stats oft. com
Strzelec M, Białek K, Spyra A (2018) Activity of beavers as an ecological factor that affects the benthos of small rivers - a case study in the Żylica River (Poland). Biologia 73:577–588. https://doi.org/10.2478/s11756-018-0073-y
Taguchi Y-H, Oono Y (2005) Relational patterns of gene expression via non-metric multidimensional scaling analysis. Bioinformatics 21:730–740
Vehkaoja M, Nummi P (2015) Beaver facilitation in the conservation of boreal anuran communities. Herpetozoa 28(75–87):1013–4425
Vinarski MV, Serbina EA (2012) Distribution and quantitative characteristics of common species of pond snails of the subgenera Peregriana and Radix (Mollusca: Gastropoda: Lymnaeidae) in waterbodies of the south of Western Siberia, Inland. Water Biology 5(2):192–198
Washko S, Roper B, Atwood TB (2019) Beavers alter stream macroinvertebrate communities in north-eastern Utah. Freshwater Biology 65:1–13. https://doi.org/10.1111/fwb.13455
Washko S, Willby N, Law A (2022) How beavers affect riverine aquatic macroinvertebrates: a review. PeerJ 10:e13180. https://doi.org/10.7717/PEERJ.13180
Whitfield CJ, Baulch HM, Chun KP, Westbrook CJ (2014) Beaver-mediated methane emission: the effects of population growth in Eurasia and the Americas. Ambio 44:7–15. https://doi.org/10.1007/s13280-014-0575-y
Willby NJ, Law A, Levanoni O, Foster G, Ecke F (2018) Rewilding wetlands: beaver as agents of within-habitat heterogeneity and the responses of contrasting biota. Philosophical Transactions of the Royal Society B 373:20170444. https://doi.org/10.1098/rstb.2017.0444
Wojton A, Kukuła K (2021) Transformation of benthic communities in forest lowland streams colonised by Eurasian beaver Castor fiber (L.). International Review of Hydrobiology 106:131–143. https://doi.org/10.1002/iroh.202002043
Wolters JW, Reitsema RE, Verdonschot RC, Schoelynck J, Verdonschot PF, Meire P (2019) Macrophyte-specific effects on epiphyton quality and quantity and resulting effects on grazing macroinvertebrates. Freshwater Biology 64(6):1131–1142
Yang L, He H, Guan B, Yu J, Yao Z, Zhen W, Liu Z (2020) Mesocosm experiment reveals a strong positive effect of snail presence on macrophyte growth, resulting from control of epiphyton and nuisance filamentous algae: implications for shallow lake management. Science of the Total Environment 705:135958. https://doi.org/10.1016/j.scitotenv.2019.135958
Zavyalov NA (2014) Beavers (Castor fiber and Castor canadensis), the founders of habitats and phytophages. Biology Bulletin Reviews 4(2):157–180. https://doi.org/10.1134/S207908641402008X
Zavyalov NA (2016) Beavers as regulators of substance and energy transfer in ecosystems of small rivers: why is it difficult to get an overall picture? Contemporary Problems of Ecology 9:481–493. https://doi.org/10.1134/S1995425516040120
Zavyalov NA, Krylov AV, Bobrov AA, Ivanov VK, Dgebuadze YY (2005) Impact of the European beaver on small river ecosystems. Nauka, Moscow [In Russian]
Zealand AM, Jeffries MJ (2009) The distribution of pond snail communities across a landscape: separating out the influence of spatial position from local habitat quality for ponds in south-east Northumberland, UK. Hydrobiologia 632(1):177–187
Acknowledgements
The authors thank Anton Svinin and Vitaly Osipov for their help in collecting material in the field; management of the Privolzhskaya Lesostep nature reserve for the opportunity to work on the territory; M.V. Vinarski for help in identifying the pond snail Ampullaceana ampla.
Funding
This work was supported by the IPEE RAS task 0089–2021-0006 “Ecology and Biodiversity of Aquatic Communities” (reg. number AAAA-A18-118042490059–5).
Author information
Authors and Affiliations
Contributions
Conceptualisation: IB. Developing methods: IB, TS. Data analysis: IB, TS. Preparation of figures and tables: IB. Conducting the research, data interpretation, writing: IB, TS.
Corresponding author
Ethics declarations
Conflict of Interest Statement
Authors claim no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bashinskiy, I.W., Stojko, T.G. The more Diverse Beaver Ponds are Better – a Case Study of Mollusc Communities of Steppe Streams. Wetlands 42, 104 (2022). https://doi.org/10.1007/s13157-022-01625-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-022-01625-8