Abstract
Purpose
Dopamine transporter imaging is suggested to be a useful imaging biomarker for Parkinson’s disease (PD) progression and monitoring drug effects. We investigated the longitudinal decline characteristics of striatal [18F]FP-CIT uptake in PD.
Methods
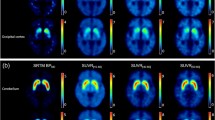
We retrospectively reviewed 35 PD patients and 9 non-PD patients. All patients underwent [18F]FP-CIT PET at the initial diagnosis and follow-up. PET images were spatially normalized and analyzed with eight striatal and one occipital VOI templates. We measured the specific to non-specific binding ratio (SNBR) of the striatal subregions and calculated the absolute annual reduction (AAR) and relative annual reduction (%RAR) of the SNBRs.
Results
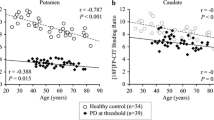
Total striatal SNBRs in PD patients were significantly lower than those in non-PD patients, with the most significant difference in the posterior putamen. Both AAR (0.26 ± 0.14 vs. 0.09 ± 0.19, p < 0.05) and %RAR (6.9 ± 3.5 vs. 1.2 ± 2.7, p < 0.001) of total striatal SNBRs were significantly greater in PD than non-PD patients. There were no significant differences in the AAR and %RAR of total striatal SNBRs between elderly and young onset PD. The AARs of the posterior putamen were higher in early PD than in advanced PD. Conversely, the %RARs were not significantly different between early and more advanced PD. The disease duration was significantly negatively correlated with the AAR but not with the %RAR of the posterior putamen.
Conclusions
The longitudinal decline of striatal [18F]FP-CIT uptake in PD was nonlinear and significantly faster than that in non-PD, with a different rate of decline among the striatal subregions.




Similar content being viewed by others
References
Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol. 2005;252(Suppl 4):iv37–42.
Oh M, Kim JS, Kim JY, Shin KH, Park SH, Kim HO, et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med. 2012;53:399–406.
Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287:1653–61.
Seibyl JP, Marek K, Sheff K, Zoghbi S, Baldwin RM, Charney DS, et al. Iodine-123-beta-CIT and iodine-123-FPCIT SPECT measurement of dopamine transporters in healthy subjects and Parkinson’s patients. J Nucl Med. 1998;39:1500–8.
Winogrodzka A, Bergmans P, Booij J, van Royen EA, Janssen AG, Wolters EC. [123I]FP-CIT SPECT is a useful method to monitor the rate of dopaminergic degeneration in early-stage Parkinson’s disease. J Neural Transm (Vienna). 2001;108:1011–9.
Marek K, Innis R, van Dyck C, Fussell B, Early M, Eberly S, et al. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology. 2001;57:2089–94.
Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301.
Brooks DJ, Salmon EP, Mathias CJ, Quinn N, Leenders KL, Bannister R, et al. The relationship between locomotor disability, autonomic dysfunction, and the integrity of the striatal dopaminergic system in patients with multiple system atrophy, pure autonomic failure, and Parkinson’s disease, studied with PET. Brain. 1990;113(Pt 5):1539–52.
Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson’s disease. Brain. 1996;119(Pt 2):585–91.
Vingerhoets FJ, Snow BJ, Lee CS, Schulzer M, Mak E, Calne DB. Longitudinal fluorodopa positron emission tomographic studies of the evolution of idiopathic parkinsonism. Ann Neurol. 1994;36:759–64.
Morrish PK, Rakshi JS, Bailey DL, Sawle GV, Brooks DJ. Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry. 1998;64:314–9.
Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000;47:493–503.
Lee SJ, Oh SJ, Chi DY, Kang SH, Kil HS, Kim JS, et al. One-step high-radiochemical-yield synthesis of [18F]FP-CIT using a protic solvent system. Nucl Med Biol. 2007;34:345–51.
Park E, Hwang YM, Lee CN, Kim S, Oh SY, Kim YC, et al. Differential diagnosis of patients with inconclusive Parkinsonian features using [(18)F]FP-CIT PET/CT. Nucl Med Mol Imaging. 2014;48:106–13.
Foundation NP. Available from: http://www.parkinson.org/understanding-parkinsons/what-is-parkinsons/young-onset-parkinsons
Kim HW, Kim JS, Oh M, Oh JS, Lee SJ, Oh SJ, et al. Different loss of dopamine transporter according to subtype of multiple system atrophy. Eur J Nucl Med Mol Imaging. 2016;43:517–25.
Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80.
Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, et al. Differing patterns of striatal 18F-dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. 1990;28:547–55.
Brooks DJ. Functional imaging in relation to parkinsonian syndromes. J Neurol Sci. 1993;115:1–17.
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55.
German DC, Manaye K, Smith WK, Woodward DJ, Saper CB. Midbrain dopaminergic cell loss in Parkinson’s disease: computer visualization. Ann Neurol. 1989;26:507–14.
Rinne JO, Rummukainen J, Paljarvi L, Rinne UK. Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Ann Neurol. 1989;26:47–50.
Nurmi E, Bergman J, Eskola O, Solin O, Vahlberg T, Sonninen P, et al. Progression of dopaminergic hypofunction in striatal subregions in Parkinson’s disease using [18F]CFT PET. Synapse. 2003;48:109–15.
Nurmi E, Ruottinen HM, Kaasinen V, Bergman J, Haaparanta M, Solin O, et al. Progression in Parkinson’s disease: a positron emission tomography study with a dopamine transporter ligand [18F]CFT. Ann Neurol. 2000;47:804–8.
Nurmi E, Bergman J, Eskola O, Solin O, Hinkka SM, Sonninen P, et al. Reproducibility and effect of levodopa on dopamine transporter function measurements: a [18F]CFT PET study. J Cereb Blood Flow Metab. 2000;20:1604–9.
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498–508.
Lee CS, Kim S-J, Oh SJ, Kim HO, Yun S-C, Doudet D, et al. Uneven age effects of [18F] FP-CIT binding in the striatum of Parkinson’s disease. Ann Nucl Med. 2014;28:874–9.
Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132(Pt 11):2947–57.
Gibb WR, Lees AJ. A comparison of clinical and pathological features of young- and old-onset Parkinson’s disease. Neurology. 1988;38:1402–6.
Staffen W, Mair A, Unterrainer J, Trinka E, Ladurner G. Measuring the progression of idiopathic Parkinson’s disease with [123I] beta-CIT SPECT. J Neural Transm (Vienna). 2000;107:543–52.
Acknowledgements
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C2768).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Changhwan Sung, Jai Hyuen Lee, Jungsu S Oh, Minyoung Oh, Sang Ju Lee, Seung Jun Oh, Sun Ju Chung, Chong Sik Lee, and Jae Seung Kim declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by our Institutional Review Board (IRB no. S2016–2189-0001).
Informed Consent
The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
Rights and permissions
About this article
Cite this article
Sung, C., Lee, J.H., Oh, J.S. et al. Longitudinal Decline of Striatal Subregional [18F]FP-CIT Uptake in Parkinson’s Disease. Nucl Med Mol Imaging 51, 304–313 (2017). https://doi.org/10.1007/s13139-017-0481-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-017-0481-x