Abstract
Purpose
Serum thyroglobulin (Tg) level is frequently elevated shortly after radioactive iodine (RAI) ablation therapy. The authors studied the relationship between the elevation of serum Tg after RAI therapy and iodine uptake pattern on post-ablation whole body scans (RxWBSs) in patients with papillary thyroid carcinoma (PTC).
Materials and Methods
The study subjects were patients with PTC that had undergone first RAI therapy with thyroid hormone withdrawal after total thyroidectomy. Patients with a high level of serum anti-Tg antibody (TgAb, ≥ 60 U/mL), possible regional or distant metastasis as determined by pre-ablation or post-ablation studies, and negative iodine uptake of the anterior neck on RxWBS were excluded. Serum Tg was checked twice, that is, 7 days after (post-ablation Tg) and on the day of RAI therapy (pre-ablation Tg). Ratio of pre-ablation Tg to post-ablation Tg (Tg ratio) was used to assess changes in serum Tg levels after RAI therapy. Patients were classified into two groups according to the presence of midline uptake above the thyroidectomy bed on RxWBS (negative (group 1) or positive (group 2) midline uptake). Variables were subjected to analysis to identify differences between the two groups.
Results
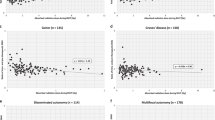
Two hundred and fifty patients were enrolled in this study; 101 in group 1 and 149 in group 2. Based on univariate analysis, post-ablation Tg (8.12 ± 11.05 vs. 34.12 ± 54.31; P < 0.001) and Tg ratio (7.81 ± 8.98 vs. 20.01 ± 19.84; P < 0.001) were significantly higher in group 2. On the other hand, gender, tumor (T) stage, lymph node (N) stage, size, multiplicity or bilaterality of primary tumor, dose of 131I, serum TgAb and thyroid-stimulating hormone (TSH) level (before or after RAI therapy) were not significantly different in the two groups. Variables with P values of < 0.25 by univariate analysis were subjected to multivariate analysis, which showed post-ablation Tg (OR 1.060, 95 % CI = 1.028–1.092; P < 0.001) and Tg ratio (OR 1.059, 95 % CI = 1.028–1.092; P = 0.001) were significantly higher in group 2.
Conclusion
Serum Tg level after RAI therapy was significantly higher in patients with midline uptake on RxWBS, compared with patients without midline uptake on RxWBS. Further investigations are needed to reveal the correlation between serum Tg elevation and clinical outcome according to the presence of midline uptake.




Similar content being viewed by others
References
Park HJ, Jeong GC, Kwon SY, et al. Stimulated serum thyroglobulin level at the time of first dose of radioactive iodine therapy is the most predictive factor for therapeutic failure in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging. 2014;48:255–61.
Lee JI, Chung YJ, Cho BY, et al. Postoperative-stimulated serum thyroglobulin measured at the time of 131I ablation is useful for the prediction of disease status in patients with differentiated thyroid carcinoma. Surgery. 2013;153:828–35.
Webb RC, Howard RS, Stojadinovic A, et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab. 2012;97:2754–63.
Kendler DB, Vaisman F, Corbo R, Martins R, Vaisman M. Pre-ablation stimulated thyroglobulin is a good predictor of successful ablation in patients with differentiated thyroid cancer. Clin Nucl Med. 2012;37:545–9.
Vaisman A, Orlov S, Yip J, et al. Application of post-surgical stimulated thyroglobulin for radioiodine remnant ablation selection in low-risk papillary thyroid carcinoma. Head Neck. 2010;32:689–98.
Sawka AM, Orlov S, Gelberg J, et al. Prognostic value of postsurgical stimulated thyroglobulin levels after initial radioactive iodine therapy in well-differentiated thyroid carcinoma. Head Neck. 2008;30:693–700.
Kim YI, Im HJ, Paeng JC, et al. Serum thyroglobulin level after radioiodine therapy (Day 3) to predict successful ablation of thyroid remnant in postoperative thyroid cancer. Ann Nucl Med. 2015;29:184–9.
Bernier MO, Morel O, Rodien P, et al. Prognostic value of an increase in the serum thyroglobulin level at the time of the first ablative radioiodine treatment in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2005;32:1418–21.
Muratet JP, Giraud P, Daver A, et al. Predicting the efficacy of first iodine-131 treatment in differentiated thyroid carcinoma. J Nucl Med. 1997;38:1362–8.
Kim K, Kim SJ, Kim IJ, et al. Clinical significance of diffuse hepatic visualization and thyroid bed uptake on post-ablative iodine-131 whole body scan in differentiated thyroid cancer. Onkologie. 2012;35:82–6.
Lee HJ, Rha SY, Jo YS, et al. Predictive value of the pre-ablation serum thyroglobulin level after thyroidectomy is combined with post-ablation 131I whole body scintigraphy for successful ablation in patients with differentiated thyroid carcinoma. Am J Clin Oncol. 2007;30:63–8.
Hindie E, Zanotti-Fregonara P, Keller I, et al. Bone metastases of differentiated thyroid cancer: impact of early 131I-based detection on outcome. Endocr Relat Cancer. 2007;14:799–807.
Pelizzo MR, Boschin IM, Toniato A, et al. Papillary thyroid microcarcinoma (PTMC): prognostic factors, management and outcome in 403 patients. Eur J Surg Oncol. 2006;32:1144–8.
Park S, Bang JI, Lee HY, Kim SE. Does (131)I radioactivity interfere with thyroglobulin measurement in patients undergoing radioactive iodine therapy with recombinant human TSH? Nucl Med Mol Imaging. 2015;49:122–6.
Nishiyama K, Kozuka T, Higashihara T, Miyauchi K, Okagawa K. Acute radiation thyroiditis. Int J Radiat Oncol Biol Phys. 1996;36:1221–4.
Cramp WA, Yatvin MB, Harms-Ringdahl M. Recent developments in the radiobiology of cellular membranes. Acta Oncol. 1994;33:945–52.
Ramakrishnan N, McClain DE, Catravas GN. Membranes as sensitive targets in thymocyte apoptosis. Int J Radiat Biol. 1993;63:693–701.
Schlumberger M, Sebagh M, De Vathaire F, et al. Thyroid iodine content and serum thyroglobulin level following external irradiation to the neck for Hodgkin’s disease. J Endocrinol Invest. 1990;13:197–203.
Jung JS, Lee SM, Kim SJ, Choi J, Han SW. Prediction of the success of thyroid remnant ablation using pre-ablative 99mTc pertechnetate scintigraphy and post-ablative dual 131I scintigraphy. Nucl Med Commun. 2015;36:38–44.
Lee M, Lee YK, Jeon TJ, et al. Frequent visualization of thyroglossal duct remnant on post-ablation 131I-SPECT/CT and its clinical implications. Clin Radiol. 2015;70:638–43.
Lee SW, Lee J, Lee HJ, et al. Enhanced scintigraphic visualization of thyroglossal duct remnant during hypothyroidism after total thyroidectomy: prevalence and clinical implication in patients with differentiated thyroid cancer. Thyroid. 2007;17:341–6.
Bachelot A, Cailleux AF, Klain M, et al. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002;12:707–11.
Brandt MP, Kloos RT, Shen DH, et al. Micro-single-photon emission computed tomography image acquisition and quantification of sodium-iodide symporter-mediated radionuclide accumulation in mouse thyroid and salivary glands. Thyroid. 2012;22:617–24.
de Jong RJ B, Rongen RJ, Lameris JS, Knegt P, Verwoerd CD. Ultrasound characteristics of thyroglossal duct anomalies. ORL J Otorhinolaryngol Relat Spec. 1993;55:299–302.
Ewing CA, Kornblut A, Greeley C, Manz H. Presentations of thyroglossal duct cysts in adults. Eur Arch Otorhinolaryngol. 1999;256:136–8.
Hilger AW, Thompson SD, Smallman LA, Watkinson JC. Papillary carcinoma arising in a thyroglossal duct cyst: a case report and literature review. J Laryngol Otol. 1995;109:1124–7.
Sprinzl GM, Koebke J, Wimmers-Klick J, Eckel HE, Thumfart WF. Morphology of the human thyroglossal tract: a histologic and macroscopic study in infants and children. Ann Otol Rhinol Laryngol. 2000;109:1135–9.
Kurt A, Ortug C, Aydar Y, Ortug G. An incidence study on thyroglossal duct cysts in adults. Saudi Med J. 2007;28:593–7.
Acknowledgments
This work was supported by a grant from the Chonnam National University Hwasun Hospital Institute for Biomedical Science (Grant No. HCRI15001-1).
The authors thank Subin Jeon for assistance in the calculation and analysis of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Geum-Cheol Jeong, Minchul Song, Hee Jeong Park, Jung-Joon Min, Hee-Seung Bom, Ki Seong Park, Sang-Geon Cho, Sae-Ryung Kang, Jahae Kim, Ho-Chun Song and Seong Young Kwon have no conflict of interest to declare.
Ethics Statement
The protocol of this study was approved by the ethics committee in our hospital, and the study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Ethics committee waived the need to obtain informed consent.
The manuscript has not been published before or is not under consideration for publication anywhere else and has been approved by all co-authors.
Rights and permissions
About this article
Cite this article
Jeong, GC., Song, M., Park, H.J. et al. Iodine Uptake Patterns on Post-ablation Whole Body Scans are Related to Elevated Serum Thyroglobulin Levels After Radioactive Iodine Therapy in Patients with Papillary Thyroid Carcinoma. Nucl Med Mol Imaging 50, 329–336 (2016). https://doi.org/10.1007/s13139-016-0421-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-016-0421-1