Abstract
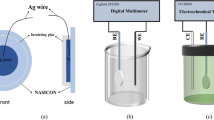
Resulting from the rising levels of atmospheric carbon, ocean acidification has become a global problem. It has significant impacts on the development, survival, growth and physiology of marine organisms. Therefore, a high-precision sensor is urgently needed to measure the pH of sea-water. Iridium wire with a diameter of 0.25 mm is used as the substrate, and an Ir/Ir(OH) x pH electrode is prepared by a one-step electrochemical method in a LiOH solution at the room temperature. A scanning electron microscope (SEM) observation reveals that it is coated with nanoscale particles. In laboratory tests, the electrode exhibits a very promising pH response, with an ideal Nernst slope (56.14–59.52), fast response, good stability and long life-span in tested pH buffer solutions. For a sea trial, four pH electrodes and one Ag/AgCl reference electrode are integrated with a self-made chemical sensor, and a profile detection of nearly 70 m is implemented near Newport Harbor, California on August 3, 2015. The results reflect that the pH value measured by the sensor is very close to the data given by Sea-Bird 911 plus CTD, with a difference value ranging from 0.000 075 to 0.064 719. And the sensor shows a better data matching degree in 0–40 m water depth. In addition, the high precision and accuracy of the sensor make it possible to use in the ocean observation field.
Similar content being viewed by others
References
Ardizzone S, Carugati A, Trasatti S. 1981. Properties of thermally prepared iridium dioxide electrodes. J Electroanal Chem Interfacial Electrochem, 126(1–3): 287–292
Bates R G, Erickson W P. 1986. Thermodynamics of the dissociation of 2-aminopyridinium ion in synthetic seawater and a standard for pH in marine systems. J Solution Chem, 15(11): 891–901
Baur J E, Spaine T W. 1998. Electrochemical deposition of iridium (IV) oxide from alkaline solutions of iridium(III) oxide. J Electroanal Chem, 443(2): 208–216
Burke L D, Whelan D P. 1984. A voltammetric investigation of the charge storage reactions of hydrous iridium oxide layers. J Electroanal Chem Interfacial Electrochem, 162(1–2): 121–141
Chen Dongchu, Li Wenfang, Huang Jinying. 2007. Preparation of a tungsten oxide pH electrochemical sensor based on solid Ag/AgCl reference electrode. Chin J Sens Actuators, 20(7): 1483–1487
Cheng Changming, Tian Xianqing, Guo Yong, et al. 2011. Large enhancement of sensitivity and a wider working range of glass pH electrode with amperometric and potentiometric responses. Electrochim Acta, 56(27): 9883–9886
Da Silva G M, Lemos S G, Pocrifka L A, et al. 2008. Development of low-cost metal oxide pH electrodes based on the polymeric precursor method. Anal Chim Acta, 616(1): 36–41
Das S, Mangwani N. 2015. Ocean acidification and marine microorganisms: responses and consequences. Oceanologia, 57(4): 349–361
Dickson A G. 1993a. pH buffers for sea water media based on the total hydrogen ion concentration scale. Deep-Sea Res: Part I. Oceanogr Res Pap, 40(1): 107–118
Dickson A G. 1993b. The measurement of sea water pH. Mar Chem, 44(2–4): 131–142
Ding Qian, Pan Yiwen, Huang Yuanfeng, et al. 2015. The optimization of Ag/Ag2S electrode using carrier electroplating of nano silver particles and its preliminary application to offshore Kueishan Tao, Taiwan. Cont Shelf Res, 111: 262–267
Feely R A, Sabine C L, Lee K, et al. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science, 305(5682): 362–366
Fog A, Buck R P. 1984. Electronic semiconducting oxides as pH sensors. Sens Actuators, 5(2): 137–146
Gimmel P, Gompf B, Schmeisser D, et al. 1989. Ta2O5-gates of pHsensitive devices: comparative spectroscopic and electrical studies. Sens Actuators, 17(1–2): 195–202
Han Chenhua, Pan Yiwen, Ye Ying. 2009. CO2 microelectrode based on Zn-Al-LDH-ion carrier and its characterization. J Trop Oceanogr (in Chinese), 28(4): 35–41
He Shichang, Zhang Yuanhui, Chen Liqi, et al. 2014. Advances in the studies of ocean acidification. Mar Sci (in Chinese), 38(6): 85–93
Huang Wending, Cao H, Deb S, et al. 2011. A flexible pH sensor based on the iridium oxide sensing film. Sens Actuators: A. Phys, 169(1): 1–11
Huang Yuanfeng, Li Jun, Yin Tianya, et al. 2015. A novel all-solidstate ammonium electrode with polyaniline and copolymer of aniline/2, 5-dimethoxyaniline as transducers. J Electroanal Chem, 741: 87–92
Huang Xiaoyan, Liu Bo, Li Xue. 2005. Applications of titanium alloys in warship building. Southern Met (in Chinese), (6): 10–11, 33
Kim T Y, Yang S. 2014. Fabrication method and characterization of electrodeposited and heat-treated iridium oxide films for pH sensing. Sens Actuators: B. Chem, 196: 31–38
Kreider K G, Tarlov M J, Cline J P. 1995. Sputtered thin-film pH electrodes of platinum, palladium, ruthenium, and iridium oxides. Sens ctuators: B. Chem, 28(3): 167–172
Kuo Limin, Chou Y C, Chen Kuanneng, et al. 2014. A precise pH microsensor using RF-sputtering IrO2 and Ta2O5 films on Pt-electrode. Sens Actuators: B. Chem, 193: 687–691
Maurya D K, Sardarinejad A, Alameh K. 2013. High-sensitivity pH sensor employing a sub-micron ruthenium oxide thin-film in conjunction with a thick reference electrode. Sens Actuators: A. Phys, 203: 300–303
McLaughlin K, Ahn J H, Litton R M, et al. 2007. Use of salinity mixing models to estimate the contribution of creek water fecal indicator bacteria to an estuarine environment: Newport Bay, California. Water Res, 41(16): 3595–3604
Olthuis W, Robben M A M, Bergveld P, et al. 1990. pH sensor properties of electrochemically grown iridium oxide. Sens Actuators: B. Chem, 2(4): 247–256
Pan Yiwen, Seyfried W E Jr. 2008. Experimental and theoretical constraints on pH measurements with an iridium oxide electrode in aqueous fluids from 25 to 175°C and 25 MPa. J Solution Chem, 37(8): 1051–1062
Prats-Alfonso E, Abad L, Casañ-Pastor N, et al. 2013. Iridium oxide pH sensor for biomedical applications. Case urea-urease in real urine samples. Biosens Bioelectron, 39(1): 163–169
Steegstra P, Ahlberg E. 2012. Influence of oxidation state on the pH dependence of hydrous iridium oxide films. Electrochim Acta, 76: 26–33
Tarlov M J, Semancik S, Kreider K G. 1990. Mechanistic and response studies of iridium oxide pH sensors. Sens Actuators: B. Chem, 1(1–6): 293–297
Wang Siru, Yin Kedong, Cai Weijun, et al. 2012. Advances in studies of ecological effects of ocean acidification. Acta Ecol Sinica (in Chinese), 32(18): 5859–5869
Whitfield M, Butler R A, Covington A K. 1985. The determination of pH in estuarine waters I. Definition of pH scales and the selection of buffers. Oceanol Acta, 8(4): 423–432
Xie Zhong, Liu Yexiang. 1998. Reference electrode for electrochemical studies in fused chloride. Chin J Nonferrous Met (in Chinese), 8(4): 668–672
Xu Bin, Zhang Weide. 2010. Modification of vertically aligned carbon nanotubes with RuO2 for a solid-state pH sensor. Electrochim Acta, 55(8): 2859–2864
Yamamoto K, Shi Guoyue, Zhou Tianshu, et al. 2003. Solid-state pH ultramicrosensor based on a tungstic oxide film fabricated on a tungsten nanoelectrode and its application to the study of endothelial cells. Anal Chim Acta, 480(1): 109–117
Yao Sheng, Wang Min, Madou M. 2001. A pH electrode based on melt-oxidized iridium oxide. J Electrochem Soc, 148(4): H29- H36
Zhao Rongrong, Xu Meizhu, Wang Jian, et al. 2010. A pH sensor based on the TiO2 nanotube array modified Ti electrode. Electrochim Acta, 55(20): 5647–5651
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: The Key Laboratory Project of State Oceanic Administration for Marine Ecosystem and Biogeochemistry of China under contract No. 529101-X21601; the Foundation from Wendy Schmidt Ocean Health XPRIZE and the Southern California Coastal Water Research Project.
Rights and permissions
About this article
Cite this article
Zhang, X., Ye, Y., Kan, Y. et al. A new electroplated Ir/Ir(OH) x pH electrode and its application in the coastal areas of Newport Harbor, California. Acta Oceanol. Sin. 36, 99–104 (2017). https://doi.org/10.1007/s13131-017-1064-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-017-1064-5