Abstract
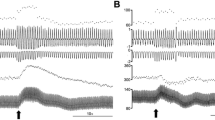
Connections between the midbrain dorsolateral periaqueductal grey (dlPAG) and the pontine A5 region have been shown. The stimulation of both regions evokes similar cardiovascular responses: tachycardia and hypertension. Accordingly, we have studied the interactions between dlPAG and A5 region in spontaneously breathing anesthetized rats. dlPAG was electrically stimulated (20–30 μA 1-ms pulses were given for 5 s at 100 Hz). Changes in the evoked cardiorespiratoy response were analysed before and after ipsilateral microinjections of muscimol (GABAergic agonist, 50 nl, 0.25 nmol, 5 s) within the A5 region. Electrical stimulation of the dlPAG produces, in the rat, a response characterized by tachypnoea (p < 0.001), hypertension (p < 0.001) and tachycardia (p < 0.001). The increase in respiratory rate was due to a decrease in expiratory time (p < 0.01). Pharmacological inhibition of the A5 region with muscimol produced a marked reduction of the tachycardia (p < 0.001) and the tachypnoea (p < 0.01) evoked from the dlPAG. Finally, to assess functional interactions between A5 and dlPAG, extracellular activity of putative A5 neurones were recorded during dlPAG electrical stimulation. Forty A5 cells were recorded, 16 of which were affected by dlPAG stimulation (40%). 4 cells showed activation, 5 cells excitation and 7 cells decreased spontaneous activity to dlPAG stimulation (p < 0.001). These results confirm a link between the A5 region and dlPAG. The potential role of these connections in the modulation of dlPAG evoked cardiorespiratory responses and their possible clinical implications are discussed.






Similar content being viewed by others
References
Bajic D, Proudfit HK (2013) Projections from the rat cuneiform nucleus to the A7, A6 (locus coeruleus), and A5 pontine noradrenergic cell groups. J Chem Neuroanat 50-51:11–20. https://doi.org/10.1016/j.jchemneu.2013.03.001
Bandler R, Keay KA, Floyd N, Price J (2000) Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 53(1):95–104. https://doi.org/10.1016/S0361-9230(00)00313-0
Benarroch EE, Schmeichel AM, Low PA, Parisi JE (2010) Differential involvement of the periaqueductal gray in multiple system atrophy. Auton Neurosci 158:111–117. https://doi.org/10.1016/j.autneu.2010.07.009
Benarroch EE, Schmeichel AM, Low PA, Sandroni P, Parisi JE (2008) Loss of A5 noradrenergic neurons in multiple system atrophy. Acta Neuropathol 115(6):629–634. https://doi.org/10.1007/s00401-008-0351-9
Bruinstroop E, Cano G, Vanderhorst VG, Cavalcante JC, Wirth J, Sena-Esteves M, Saper CB (2012) Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. J Comp Neurol 520:1985–2001. https://doi.org/10.1002/cne.23024
Carrive P (1993) The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res 58:27–47. https://doi.org/10.1016/0166-4328(93)90088-8
Comet MA, Sevoz-Couche C, Hanoun N, Hamon M, Laguzzi R (2004) 5-HT-mediated in-hibition of cardiovagal baroreceptor reflex response during defense reaction in the rat. Am J Physiol Heart Circ Physiol 287(4):H1641e9
Da Silva LG, de Menezes RCA, Santos RAS, Campagnole-Santos MJ, Fontes MAP (2003) Role of periaqueductal gray on the cardiovascular response evoked by disinhibition of the dorsomedial hypothalamus. Brain Res 984:206–214. https://doi.org/10.1016/S0006-8993(03)03157-3
Dampney RAL (2015) Central mechanisms regulating coordinated cardiovascular and respiratory function during stress and arousal. Am J Physiol Regul Integr Comp Physiol 309:R429–R443. https://doi.org/10.1152/ajpregu.00051
Dampney RAL, Furlong TM, Horiuchi J, Iigaya K (2013) Role of dorsolateral periaqueductal grey in the coordinated regulation of cardiovascular and respiratory function. Auton Neurosci 175(1–2):17–25. https://doi.org/10.1016/j.autneu.2012.12.008
Dawid Milner MS, Lara JP, Lopez de Miguel MP, Lopez-Gonzalez MV, Spyer KM, Gonzalez-Baron S (2003) A5 region modulation of the cardiorespiratory responses evoked from parabrachial cell bodies in the anaesthetised rat. Brain Res 982:108–118. https://doi.org/10.1016/S0006-8993(03)03005-1
Dawid-Milner MS, Lara JP, Gonzalez-Baron S, Spyer KM (2001) Respiratory effects of stimulation of cell bodies of the A5 region in the anaesthetised rat. Pflugers Arch 441:434–443. https://doi.org/10.1007/s004240000450
Diaz-Casares A, Lopez-Gonzalez MV, Peinado-Aragones CA, Gonzalez-Baron S, Dawid-Milner MS (2012) Parabrachial complex glutamate receptors modulate the cardiorespiratory response evoked from hypothalamic defense area. Auton Neurosci 169:124–134. https://doi.org/10.1016/j.autneu.2012.06.001
Díaz-Casares A, López-González MV, Peinado-Aragonés CA, Lara JP, González-Barón S, Dawid-Milner MS (2009) Role of the parabrachial complex in the cardiorespiratory response evoked from hypothalamic defense area stimulation in the anesthetized rat. Brain Res 1279:58–70. https://doi.org/10.1016/j.brainres.2009.02.085
Goodchild AK, Phillips JK, Lipski J, Pilowsky PM (2001) Differential expression of catecholamine synthetic enzymes in the caudal ventral pons. J Comp Neurol 438(4):457–467. https://doi.org/10.1002/cne.1328
Guyenet PG, Stornetta RL, Bayliss DA (2010) Central respiratory chemoreception. J Comp Neurol 518(19):3883–3906. https://doi.org/10.1002/cne.22435
Guyenet PG (2006) The sympathetic control of blood pressure. Nat Rev Neurosci 7:335–346
Guyenet PG, Koshiya N, Huangfu D, Verberne AJ, Riley TA (1993) Central respiratory control of A5 and A6 pontine noradrenergic neurons. Am J Phys 264:1035–1044
Hayward LF, Castellanos M, Davenport PW (2004) Parabrachial neurons mediate dorsal periaqueductal gray evoked respiratory responses in the rat. J Appl Physiol 96(3):1146–1154. https://doi.org/10.1152/japplphysiol.00903
Hayward LF, Von Reitzenstein M (2002) c-Fos expression in the midbrain periaqueductal gray after chemoreceptor and baroreceptor activation. Am J Physiol Heart Circ Physiol 283:1975–1984. https://doi.org/10.1152/ajpheart.00300
Hilaire G (2006) Endogenous noradrenaline affects the maturation and function of the respiratory network: possible implication for SIDS. Auton Neurosci 126-127:320–331. https://doi.org/10.1016/j.autneu.2006.01.021
Horiuchi J, McDowall LM, Dampney RAL (2009) Vasomotor and respiratory responses evoked from the dorsolateral periaqueductal grey are mediated by the dorsomedial hypothalamus. J Physiol 587:5149–5162. https://doi.org/10.1113/jphysiol.2009.179739
Horiuchi J, McDowall LM, Dampney RA (2006) Differential control of cardiac and sympathetic vasomotor activity from the dorsomedial hypothalamus. Clin Exp Pharmacol Physiol 33(12):1265–1268
Huangfu DH, Koshiya N, Guyenet PG (1991) A5 noradrenergic unit activity and sympathetic nerve discharge in rats. Am J Phys 261:393–402. https://doi.org/10.1152/ajpregu.1991.261.2.R393
Jodkowski JS, Coles SK, Dick TE (1994) A ‘pneumotaxic centre’ in rats. Neurosci Lett 172:67–72. https://doi.org/10.1016/0304-3940(94)90664-5
Kanbar R, Depuy SD, West GH, Stornetta RL, Guyenet PG (2011) Regulation of visceral sympathetic tone by A5 noradrenergic neurons in rodents. J Physiol 589:903–917. https://doi.org/10.1113/jphysiol.2010.198374
Keay KA, Bandler R (2001) Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 25:669–678. https://doi.org/10.1016/S0149-7634(01)00049-5
Koshiya N, Guyenet PG (1994) A5 noradrenergic neurons and the carotid sympathetic chemoreflex. Am J Phys 267:519–526. https://doi.org/10.1152/ajpregu.1994.267.2.R519
Krout KE, Jansen AS, Loewy AD (1998) Periaqueductal gray matter projection to the parabrachial nucleus in rat. J Comp Neurol 401:437–454. https://doi.org/10.1002/(SICI)1096-9861(19981130)401:4<437::AID-CNE2>3.0.CO;2-5
Lara JP, Dawid-Milner MS, López MV, Montes C, Spyer KM, González-Barón (2002) Laryngeal effects of stimulation of rostral and ventral pons in the anaesthetized rat. Brain Research 934:97–106. https://doi.org/10.1016/s0006-8993(02)02364-8
Loewy AD (1991) Forebrain nuclei involved in autonomic control. Prog Brain Res 87:253–268. https://doi.org/10.1016/S0079-6123(08)63055-1
López-González MV, Díaz-Casares A, González-García M, Peinado-Aragonés CA, Barbancho MA, Carrillo de Albornoz M, Dawid-Milner MS (2018) Glutamate receptors of the A5 region modulate cardiovascular responses evoked from the dorsomedial hypothalamic nucleus and perifornical area. J Physiol Biochem 74:325–334. https://doi.org/10.1007/s13105-018-0612-6
López-González MV, Díaz-Casares A, Peinado-Aragonés CA, Lara JP, Barbancho MA, Dawid-Milner MS (2013) Neurons of the A5 region are required for the tachycardia evoked by electrical stimulation of the hypothalamic defence area in anaesthetized rats. Exp Physiol 98(8):1279–1294. https://doi.org/10.1113/expphysiol.2013.072538
Netzer F, Bernard JF, Verberne AJ, Hamon M, Camus F, Benoliel JJ, Sévoz-Couche C (2011) Brain circuits mediating baroreflex bradycardia inhibition in rats: an anatomical and functional link between the cuneiform nucleus and the periaqueductal grey. J Physiol 589(8):2079–2091. https://doi.org/10.1113/jphysiol.2010.203737
Rosin DL, Chang DA, Guyenet PG (2006) Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499:64–89. https://doi.org/10.1002/cne.21105
Schlenker EH, Prestbo A (2003) Elimination of the post-hypoxic frequency decline in conscious rats lesioned in pontine A5 region. Respir Physiol Neurobiol 138:179–191
Sévoz-Couche C (2019) 5-HT3 receptor mediated neural transmission of cardiorespiratory modulation by the nucleus of the tractus solitarius. In: Pilowsky PM (ed) Serotonin. Elsevier, Amsterdam, pp 349–367. https://doi.org/10.1016/B978-0-12-800050-2.00017-6
Silva-Carvalho L, Dawid-Milner MS, Spyer KM (1995) The pattern of excitatory inputs to the nucleus tractus solitarii evoked on stimulation in the hypothalamic defence area in the cat. J Physiol 487(3):727–737. https://doi.org/10.1113/jphysiol.1995.sp020915
Tavares I, Lima D (2002) The caudal ventrolateral medulla as an important inhibitory modulator of pain transmission in the spinal cord. J Pain 3(5):337–346. https://doi.org/10.1054/jpai.2002.127775
Tavares I, Lima D, Coimbra A (1996) The ventrolateral medulla of the rat is connected with the spinal cord dorsal horn by an indirect descending pathway relayed in the A5 noradrenergic cell group. J Comp Neurol 374:84–95. https://doi.org/10.1002/(SICI)1096-9861(19961007)374:1<84::AID-CNE6>3.0.CO;2-J
Taxini CL, Moreira TS, Takakura AC, Bícego KC, Gargaglioni LH, Zoccal DB (2017) Role of A5 noradrenergic neurons in the chemoreflex control of respiratory and sympathetic activities in unanesthetized conditions. Neuroscience 354:146–157. https://doi.org/10.1016/j.neuroscience.2017.04.033
Taxini CL, Takakura AC, Gargaglioni LH, Moreira TS (2011) Control of the central chemoreflex by A5 noradrenergic neurons in rats. Neuroscience 199:177–186. https://doi.org/10.1016/j.neuroscience.2011.09.068
Vianna DM, Brandão ML (2003) Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear. Braz J Med Biol Res 36(5):557–566. https://doi.org/10.1590/S0100-879X2003000500002
Funding
The study was supported by a program grant Junta de Andalucía, Group no. CTS-156, Spain. Part of the final study was supported with a grant, PPit 2017/14, from the Own Funds Program of the University of Málaga.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experimental protocols were performed in accordance with the recommendations of the European Union directive (2010/63/EU) for animal care and experimental procedures. The experiments were approved by the Ethical Committee for Animal Research of the University of Malaga and the Junta de Andalucía. Every attempt was made to reduce animal suffering, discomfort and the number of animals needed to obtain reliable results.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
- A5 region inhibition reduces dlPAG evoked tachycardic and tachypnoeic responses.
- Some A5 cells recorded were affected by dlPAG stimulation.
- A5 region and dlPAG are shown to be functionally related.
Díaz-Casares and Dawid-Milner both had equally responsibility and must be considered last authors.
Rights and permissions
About this article
Cite this article
López-González, M.V., González-García, M., Peinado-Aragonés, C.A. et al. Pontine A5 region modulation of the cardiorespiratory response evoked from the midbrain dorsolateral periaqueductal grey. J Physiol Biochem 76, 561–572 (2020). https://doi.org/10.1007/s13105-020-00761-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-020-00761-1