Abstract
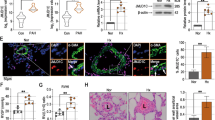
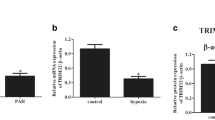
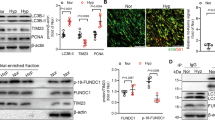
Increased evidence indicates that adenosine monophosphate-activated protein kinase (AMPK) plays a vital role in vascular homeostasis, especially under hypoxia, and protects against the progression of pulmonary hypertension (PH). However, the role of AMPK in the pathogenesis of PH remains to be clarified. In the present study, we confirmed that a loss of AMPKα2 exacerbated the development of PH by using hypoxia-induced PH model in AMPKα2 −/− mice. After a 4-week period of hypoxic exposure, AMPKα2 −/− mice exhibited more severe pulmonary vascular remodeling and pulmonary vascular smooth muscle cell (SMC) proliferation when compared with wild type (WT) mice. In vitro, AMPKα2 knockdown promoted the proliferation of pulmonary arterial smooth muscle cells (PASMCs) under hypoxia. This phenomenon was accompanied by upregulated Skp2 and downregulated p27kip1 expression and was abolished by rapamycin, an inhibitor of mTOR. These results indicate that AMPKα2 deficiency exacerbates hypoxia-induced PH by promoting PASMC proliferation via the mTOR/Skp2/p27kip1 signaling axis. Therefore, enhanced AMPKα2 activity might underlie a novel therapeutic strategy for the management of PH.






Similar content being viewed by others
References
Auld CA, Fernandes KM, Morrison RF (2007) Skp2-mediated p27(Kip1) degradation during S/G2 phase progression of adipocyte hyperplasia. J Cell Physiol 211:101–111. https://doi.org/10.1002/jcp.20915
Bond M, Wu YJ (2011) Proliferation unleashed: the role of Skp2 in vascular smooth muscle cell proliferation. Front Biosci (Landmark edition) 16:1517–1535
Cetrullo S, D’Adamo S, Tantini B, Borzi RM, Flamigni F (2015) mTOR, AMPK, and Sirt1: key players in metabolic stress management. Crit Rev Eukaryot Gene Expr 25:59–75
Chen H, Mo X, Yu J, Huang S, Huang Z, Gao L (2014) Interference of Skp2 effectively inhibits the development and metastasis of colon carcinoma. Mol Med Rep 10:1129–1135. https://doi.org/10.3892/mmr.2014.2308
Conradie R, Bruggeman FJ, Ciliberto A, Csikasz-Nagy A, Novak B, Westerhoff HV, Snoep JL (2010) Restriction point control of the mammalian cell cycle via the cyclin E/Cdk2:p27 complex. FEBS J 277:357–367. https://doi.org/10.1111/j.1742-4658.2009.07473.x
Council NR (2011) Guide for the care and use of laboratory animals. In: 8th (ed) Guide for the care and use of laboratory animals. National Academies Press, Washington (DC). https://doi.org/10.17226/12910
Dazert E, Hall MN (2011) mTOR signaling in disease. Curr Opin Cell Biol 23:744–755. https://doi.org/10.1016/j.ceb.2011.09.003
Fisslthaler B, Fleming I (2009) Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105:114–127. https://doi.org/10.1161/CIRCRESAHA.109.201590
Fujita T, Liu W, Doihara H, Wan Y (2008) Regulation of Skp2-p27 axis by the Cdh1/anaphase-promoting complex pathway in colorectal tumorigenesis. Am J Pathol 173:217–228. https://doi.org/10.2353/ajpath.2008.070957
Hardie DG (2011) AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 25:1895–1908. https://doi.org/10.1101/gad.17420111
Ibe JC, Zhou Q, Chen T, Tang H, Yuan JX, Raj JU, Zhou G (2013) Adenosine monophosphate-activated protein kinase is required for pulmonary artery smooth muscle cell survival and the development of hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol 49:609–618. https://doi.org/10.1165/rcmb.2012-0446OC
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955–968. https://doi.org/10.1016/j.cell.2006.06.055
Jiang W, Zhu Z, Thompson HJ (2008) Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res 68:5492–5499. https://doi.org/10.1158/0008-5472.can-07-6721
Ke R, Liu L, Zhu Y, Li S, Xie X, Li F, Song Y, Yang L, Gao L, Li M (2016) Knockdown of AMPKalpha2 promotes pulmonary arterial smooth muscle cells proliferation via mTOR/Skp2/p27(Kip1) signaling pathway. Int J Mol Sci 17. https://doi.org/10.3390/ijms17060844
Kopsiaftis S, Sullivan KL, Garg I, Taylor JA 3rd, Claffey KP (2016) AMPKalpha2 regulates bladder cancer growth through SKP2-mediated degradation of p27. Mol Cancer Res 14:1182–1194. https://doi.org/10.1158/1541-7786.mcr-16-0111
Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT (2014) Pulmonary arterial hypertension: the clinical syndrome. Circ Res 115:115–130. https://doi.org/10.1161/CIRCRESAHA.115.301146
Lai YC, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, St Croix CM, Garcia-Ocana A, Goncharova EA, Tofovic SP, Mora AL, Gladwin MT (2016) SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation 133:717–731. https://doi.org/10.1161/circulationaha.115.018935
Larrea MD, Wander SA, Slingerland JM (2009) p27 as Jekyll and Hyde: regulation of cell cycle and cell motility. Cell Cycl (Georgetown, Tex) 8:3455–3461. https://doi.org/10.4161/cc.8.21.9789
McLaughlin VV, Shah SJ, Souza R, Humbert M (2015) Management of pulmonary arterial hypertension. J Am Coll Cardiol 65:1976–1997. https://doi.org/10.1016/j.jacc.2015.03.540
Ogawa A, Firth AL, Yao W, Madani MM, Kerr KM, Auger WR, Jamieson SW, Thistlethwaite PA, Yuan JX (2009) Inhibition of mTOR attenuates store-operated Ca2+ entry in cells from endarterectomized tissues of patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 297:L666–L676. https://doi.org/10.1152/ajplung.90548.2008
Omura J, Satoh K, Kikuchi N, Satoh T, Kurosawa R, Nogi M, Otsuki T, Kozu K, Numano K, Suzuki K, Sunamura S, Tatebe S, Aoki T, Sugimura K, Miyata S, Hoshikawa Y, Okada Y, Shimokawa H (2016) Protective roles of endothelial AMP-activated protein kinase against hypoxia-induced pulmonary hypertension in mice. Circ Res 119:197–209. https://doi.org/10.1161/circresaha.115.308178
Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA (2008) Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3:1422–1434. https://doi.org/10.1038/nprot.2008.138
Ray A, James MK, Larochelle S, Fisher RP, Blain SW (2009) p27Kip1 inhibits cyclin D-cyclin-dependent kinase 4 by two independent modes. Mol Cell Biol 29:986–999. https://doi.org/10.1128/mcb.00898-08
Salt IP, Hardie DG (2017) AMP-activated protein kinase: an ubiquitous signaling pathway with key roles in the cardiovascular system. Circ Res 120:1825–1841. https://doi.org/10.1161/CIRCRESAHA.117.309633
Song P, Wang S, He C, Wang S, Liang B, Viollet B, Zou MH (2011) AMPKalpha2 deletion exacerbates neointima formation by upregulating Skp2 in vascular smooth muscle cells. Circ Res 109:1230–1239. https://doi.org/10.1161/circresaha.111.250423
Song Y, Wu Y, Su X, Zhu Y, Liu L, Pan Y, Zhu B, Yang L, Gao L, Li M (2016) Activation of AMPK inhibits PDGF-induced pulmonary arterial smooth muscle cells proliferation and its potential mechanisms. Pharmacol Res 107:117–124. https://doi.org/10.1016/j.phrs.2016.03.010
Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM (2012) Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 186:261–272. https://doi.org/10.1164/rccm.201201-0164OC
Sutendra G, Michelakis ED (2013) Pulmonary arterial hypertension: challenges in translational research and a vision for change. Sci Transl Med 5:208sr205. https://doi.org/10.1126/scitranslmed.3005428
Tajsic T, Morrell NW (2011) Smooth muscle cell hypertrophy, proliferation, migration and apoptosis in pulmonary hypertension. Compr Physiol 1:295–317. https://doi.org/10.1002/cphy.c100026
Totary-Jain H, Sanoudou D, Dautriche CN, Schneller H, Zambrana L, Marks AR (2012) Rapamycin resistance is linked to defective regulation of Skp2. Cancer Res 72:1836–1843. https://doi.org/10.1158/0008-5472.can-11-2195
Vila IK, Yao Y, Kim G, Xia W, Kim H, Kim SJ, Park MK, Hwang JP, Gonzalez-Billalabeitia E, Hung MC, Song SJ, Song MS (2017) A UBE2O-AMPKalpha2 axis that promotes tumor initiation and progression offers opportunities for therapy. Cancer Cell 31:208–224. https://doi.org/10.1016/j.ccell.2017.01.003
Zang Y, Yu LF, Nan FJ, Feng LY, Li J (2009) AMP-activated protein kinase is involved in neural stem cell growth suppression and cell cycle arrest by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside and glucose deprivation by down-regulating phospho-retinoblastoma protein and cyclin D. J Biol Chem 284:6175–6184. https://doi.org/10.1074/jbc.M806887200
Zhan JK, Wang YJ, Wang Y, Tang ZY, Tan P, Huang W, Liu YS (2014) Adiponectin attenuates the osteoblastic differentiation of vascular smooth muscle cells through the AMPK/mTOR pathway. Exp Cell Res 323:352–358. https://doi.org/10.1016/j.yexcr.2014.02.016
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35. https://doi.org/10.1038/nrm3025
Acknowledgments
The authors thank the Department of Cardiovascular Surgery, Xinqiao Hospital, Army Medical University, and Institute of Respiratory Diseases, Xinqiao Hospital, Army Medical University, for their support for this study.
Funding
This study was funded by the National Natural Science Foundation of China (grant nos. 81370004 and 81270228).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
1. AMPKα2 knockout exacerbates hypoxia-induced pulmonary hypertension (PH) in mice.
2. AMPKα2 deficiency promotes PASMC proliferation under hypoxia condition.
3. mTOR activation mediates the effect of AMPKα2 on PASMC proliferation.
Rights and permissions
About this article
Cite this article
Wang, HL., Tang, FQ., Jiang, YH. et al. AMPKα2 deficiency exacerbates hypoxia-induced pulmonary hypertension by promoting pulmonary arterial smooth muscle cell proliferation. J Physiol Biochem 76, 445–456 (2020). https://doi.org/10.1007/s13105-020-00742-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-020-00742-4