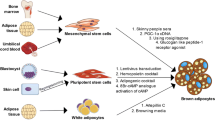
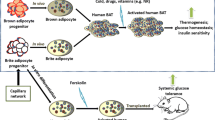
Abstract
Recent investigations have showed that the functional thermogenic adipocytes are present in both infants and adult humans. Accumulating evidence suggests that the coexistence of classical and inducible brown (brite) adipocytes in humans at adulthood and these adipocytes function to generate heat from energy resulting in reducing body fat and improving glucose metabolism. Human thermogenic adipocytes can be differentiated in vitro from stem cells, cell lines, or adipose stromal vascular fraction. Pre-activated human brite adipocytes in vitro can maintain their thermogenic function in normal or obese immunodeficient mice; therefore, they improve glucose homeostasis and reduce fat mass in obese animals. These key findings have opened a new door to use in vitro thermogenic adipocytes as a cell therapy to prevent obesity and related disorders. Thus, this paper intends to highlight our knowledge in aspects of in vitro human brite/brown adipocytes for the further studies.

Similar content being viewed by others
Change history
05 October 2017
Volume 73 issue 3 was published with an incorrect cover date. Correct is August 2017. The Publisher apologizes for this mistake and all related inconveniences caused by this.
References
Atit R et al (2006) β-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 296(1):164–176
Barbara C, Nedergaarb J (2004) Brown adipose tissue: function and physiological significance. Physiol rev 84(1):277–359
Barclay JL et al (2015) Effects of glucocorticoids on human brown adipocytes. J Endocrinol 224(2):139–147
Barquissau V et al (2016) White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Molecular Metabolism 5(5):352–365
Bordicchia M et al (2012) Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 122(3):1022–1036
Brydon L et al (2001) Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology 142(10):4264–4271
Cantó C et al (2012) The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15(6):838–847
Chu D-T, Tao Y (2017) Human thermogenic adipocytes: a reflection on types of adipocyte, developmental origin, and potential application. J Physiol Biochem 73(1):1–4
Chu D-T et al (2014) Expression of adipocyte biomarkers in a primary cell culture models reflects preweaning adipobiology. J Biol Chem 289(26):18478–18488
Chu D-T, Tao Y, Taskén K (2017a) OPA1 in lipid metabolism: function of OPA1 in lipolysis and thermogenesis of adipocytes. Horm Metab res 49(4):276–285
Chu D-T et al (2017b) C57BL/6J mice as a polygenic developmental model of diet-induced obesity. Physiological Reports 5(7):e13093
Chu-Dinh T, Chu DT (2014) 4-1BB and the epigenetic regulations of this molecule. Medical Epigenetics 2(3):80–85
Cunningham S et al (1985) The characterization and energetic potential of brown adipose tissue in man. Clin Sci 69(3):343–348
Cypess AM et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J med 360(15):1509–1517
Cypess AM et al (2013) Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat med 19(5):635–639
Cypess AM et al (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21(1):33–38
Digby JE et al (1998) Thiazolidinedione exposure increases the expression of uncoupling protein 1 in cultured human preadipocytes. Diabetes 47(1):138–141
Elabd C et al (2009) Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells 27(11):2753–2760
Fischer-Posovszky P et al (2008) Human SGBS cells—a unique tool for studies of human fat cell biology. Obesity Facts 1(4):184–189
Fu T et al (2014) MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol Cell Biol 34(22):4130–4142
Gesta S, Tseng Y-H, Kahn CR (2007) Developmental origin of fat: tracking obesity to its source. Cell 131(2):242–256
Ghandour RA et al (2016) IP-receptor and PPARs trigger the conversion of human white to brite adipocyte induced by carbaprostacyclin. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1861(4):285–293
Giroud M et al (2016) Let-7i-5p represses brite adipocyte function in mice and humans. Scientific Reports 6:28613
Guennoun A et al (2015) Comprehensive molecular characterization of human adipocytes reveals a transient brown phenotype. J Transl med 13(1):135
Hafner A-L et al (2016) Brown-like adipose progenitors derived from human induced pluripotent stem cells: identification of critical pathways governing their adipogenic capacity. Scientific Reports 6:32490
Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat med 19(10):1252–1263
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nature reviews. Molecular Cell Biology 13(4):225–238
Jespersen, Naja Z., et al., A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab, 2013. 17(5): p. 798–805.
Jockers R et al (1998) Desensitization of the β-adrenergic response in human brown adipocytes. Endocrinology 139(6):2676–2684
Jukarainen S et al (2015) Obesity is associated with low NAD+/SIRT pathway expression in adipose tissue of BMI-discordant monozygotic twins. The Journal of Clinical Endocrinology & Metabolism 101(1):275–283
Jura M et al (2016) Mest and Sfrp5 are biomarkers for healthy adipose tissue. Biochimie 124:124–133
Khan NA et al (2014) Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Molecular Medicine 6(6):721–731
Klaus S et al (1995) Functional assessment of white and brown adipocyte development and energy metabolism in cell culture. Dissociation of terminal differentiation and thermogenesis in brown adipocytes. J Cell Sci 108(10):3171–3180
Klepac K et al (2016) The Gq signalling pathway inhibits brown and beige adipose tissue. Nat Commun 7:10895
Koppen A, Kalkhoven E (2010) Brown vs white adipocytes: the PPARγ coregulator story. FEBS Lett 584(15):3250–3259
Lee P et al (2011) Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology 152(10):3597–3602
Lee P et al (2014a) Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 19(2):302–309
Lee P et al (2014b) Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes 38(2):170–176
Lee MH et al (2016a) ECM microenvironment unlocks brown adipogenic potential of adult human bone marrow-derived MSCs. Scientific Reports 6:21173
Lee C-W, Hsiao W-T, Lee OK-S (2016b) Mesenchymal stromal cell-based therapies reduce obesity and metabolic syndromes induced by a high-fat diet. Transl res 182:61–74.e8
Lefterova MI et al (2014) PPARγ and the global map of adipogenesis and beyond. Trends in Endocrinology & Metabolism 25(6):293–302
Lidell ME et al (2013) Evidence for two types of brown adipose tissue in humans. Nat med 19(5):631–634
Loft A et al (2015) Browning of human adipocytes requires KLF11 and reprogramming of PPARγ superenhancers. Genes dev 25:1281–1294
Min SY et al (2016) Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat med 22(3):312–318
Mohsen-Kanson T et al (2014) Differentiation of human induced pluripotent stem cells into brown and ahite adipocytes: role of Pax3. Stem Cells 32(6):1459–1467
Okla M et al (2015) BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes. Lipids 50(2):111–120
Peirce V, Carobbio S, Vidal-Puig A (2014) The different shades of fat. Nature 510(7503):76–83
Petrovic N et al (2010) Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285(10):7153–7164
Pisani D et al (2011) Differentiation of human adipose-derived stem cells into “brite” (brown-in-white) adipocytes. Front Endocrinol 2:87
Rodriguez A-M et al (2004) Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys res Commun 315(2):255–263
Saely CH, Geiger K, Drexel H (2012) Brown versus white adipose tissue: a mini-review. Gerontology 58(1):15–23
Saito M et al (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58(7):1526–1531
Sanchez-Gurmaches J, Guertin DA (2014a) Adipocyte lineages: tracing back the origins of fat. Biochim Biophys Acta (BBA) - Mol Basis dis 1842(3):340–351
Sanchez-Gurmaches J, Guertin DA (2014b) Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun 5:4099
Seale P et al (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454(7207):961–967
Shinoda K et al (2015) Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat med 21(4):389–394
Silva FJ et al (2014) Metabolically active human brown adipose tissue derived stem cells. Stem Cells 32(2):572–581
Timmons JA et al (2007) Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci 104(11):4401–4406
van Marken Lichtenbelt WD et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J med 360(15):1500–1508
Virtanen KA et al (2009) Functional brown adipose tissue in healthy adults. N Engl J med 360(15):1518–1525
Wang Y-L et al (2016) Concomitant beige adipocyte differentiation upon induction of mesenchymal stem cells into brown adipocytes. Biochem Biophys res Commun 478(2):689–695
Wu J et al (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150(2):366–376
Zilberfarb V et al (1997) Human immortalized brown adipocytes express functional beta3-adrenoceptor coupled to lipolysis. J Cell Sci 110(7):801
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
An erratum to this article is available at https://doi.org/10.1007/s13105-017-0593-x.
Rights and permissions
About this article
Cite this article
Chu, DT., Tao, Y., Son, L.H. et al. Cell source, differentiation, functional stimulation, and potential application of human thermogenic adipocytes in vitro. J Physiol Biochem 73, 315–321 (2016). https://doi.org/10.1007/s13105-017-0567-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-017-0567-z