Abstract
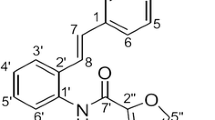
Resveratrol is identified as a natural cancer chemoprevention agent. There has been a lot of interest in designing and developing resveratrol analogs with cancer chemoprevention activity superior to that of parent molecule and exploring their action mechanism in the past several decades. In this study, we have synthesized resveratrol analogs of compounds A–C via conjugated chain elongation based on isoprene unit retention strategy. Remarkably, cytotoxic activity analysis results indicated that compound B possesses the best proliferation inhibition activity for NCI-H460 cells in all the test compounds. Intriguingly, compound B displayed a higher cytotoxicity against human non-small cell lung cancer cells (NCI-H460) compared to normal human embryonic lung fibroblasts (MRC-5). Afterward, flow cytometry analysis showed that compound B would induce cell apoptosis. We further researched the action mechanism. When NCI-H460 cells were incubated by compound B for 6 or 9 h, respectively, the intracellular reactive oxygen species (ROS) level was enhanced obviously. With elevation of intracellular ROS level, flow cytometry measurement verified mitochondrial transmembrane potential collapse, which was accompanied by the up-regulation of Bax and down-regulation of Bcl-2. More interestingly, compound B increased the expression of caspase-9 and caspase-3, which induced cell apoptosis. Moreover, compound B arrested cell cycle in G0/G1 phase. These are all to provide useful information for designing resveratrol-based chemoprevention agent and understanding the action mechanism.




Similar content being viewed by others
References
Chen G, Shan W, Wu Y, Ren L, Dong J, Ji Z (2005) Structure modification of resveratrol and pharmacological activity. Chem Pharm Bull 53:1587–1590
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–762
Dai F, Liu GY, Li Y, Yan WJ, Wang Q, Yang J, Lu DL, Ding DJ, Lin D, Zhou B (2015) Insights into the importance for designing curcumin-inspired anticancer agents by a prooxidant strategy: the case of diarylpentanoids. Free Radic Biol Med 85:127–137
Hsieh TC, Wu JM (1999) Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp Cell Res 249:109–115
Hu YP, Moraes CT, Savaraj N, Priebe W, Lampidis T (2000) Rho(0) tumor cells: a model for studying whether mitochondria are targets for rhodamine 123, doxorubicin, and other drugs. J Biochem Pharmacol 60:1897–1905
Hussain RF, Nouri AME, Oliver RTD (1993) A new approach for measurement of cytotoxicity using colorimetric assay. J Immunol Methods 160:89–96
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HHS, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Kang YF, Yan WJ, Zhou TW, Dai F, Li XZ, Bao XZ, Du YT, Yuan CH, Wang HB, Ren XR, Liu Q, Jin XL, Zhou B, Zhang J (2014) Tailoring 3,3′-dihydroxyisorenieratene to hydroxystilbene: finding a resveratrol analog with the increased antiproliferation activity and cell selectivity. Chem Eur J 20:8904–8908
Krishan A (1975) Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol 66:188–193
Lecoeur H, Prévost MC, Gougeon ML (2001) Oncosis is associated with exposure of phosphatidylserine residues on the outside layer of the plasma membrane: a reconsideration of the specificity of the annexin V/propidium iodide assay. Cytometry 44:65–72
Lee SK, Zhang W, Sanderson BJS (2008) Selective growth inhibition of human leukemia and human lymphoblastoid cells by resveratrol via cell cycle arrest and apoptosis induction. J Agric Food Chem 56:7572–7577
Liu H, Dong A, Gao C, Tan C, Xie Z, Zu X, Qu L, Jiang Y (2010) New synthetic flavone derivatives induce apoptosis of hepatocarcinoma cells. Bioorg Med Chem 18:6322–6328
Niizuma K, Endo H, Chan PH (2009) Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem 109:133–138
Pailee P, Sangpetsiripan S, Mahidol C, Ruchirawat S, Prachyawarakorn V (2015) Cytotoxic and cancer chemopreventive properties of prenylated stilbenoids from Macaranga siamensis. Tetrahedron 71:5562–5571
Pervaiz S, Holme AL (2009) Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal 11:2851–2857
Roset R, Ortet L, Gil-Gómez G (2007) Role of Bcl-2 family members on apoptosis: what we have learned from knock-out mice. Front Biosci 12:4722–4730
Scherzberg MC, Kiehl A, Zivkovic A, Stark H, Stein J, Fürst R, Steinhilber D, Ulrich-Rückert S (2015) Structural modification of resveratrol leads to increased anti-tumor activity, but causes profound changes in the mode of action. Toxicol Appl Pharmacol 287:67–76
Schumacker PT (2006) Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 11:777–790
Sentürker S, Tschirret-Guth R, Morrow J, Levine R, Shacter E (2002) Induction of apoptosis by chemotherapeutic drugs without generation of reactive oxygen species. Arch Biochem Biophys 397:262–272
Shakibaei M, Harikumar KB, Aggarwal BB (2009) Resveratrol addiction: to die or not to die. Mol Nutr Food Res 53:115–128
Shih YT, Chen PS, Wu CH, Tseng YT, Wu YC, Lo YC (2010) Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free Radic Biol Med 49:1471–1479
Tang JJ, Fan GJ, Dai F, Ding DJ, Wang Q, Lu DL, Li RR, Li XZ, Hu LM, Jin XL, Zhou B (2011) Finding more active antioxidants and cancer chemoprevention agents by elongation the conjugated links of resveratrol. Free Radic Biol Med 50:1447–1457
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374
Wenzel E, Somoza V (2005) Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res 49:472–481
Acknowledgments
This work was supported by the Starting Foundation for Doctor of Hebei North University and College Young Talent Program Funding of Hebei Province (BJ2016003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, YF., Qiao, HX., Xin, LZ. et al. Chain elongation analog of resveratrol as potent cancer chemoprevention agent. J Physiol Biochem 72, 445–452 (2016). https://doi.org/10.1007/s13105-016-0487-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0487-3