Abstract
Robust postoperative bypass development is a characteristic of moyamoya disease (MMD); however, genetic factors mediating this phenomenon remain incompletely understood. Therefore, we aimed to elucidate the relationship between postoperative donor artery development and genetic variants. We retrospectively enrolled 63 patients (79 hemispheres) who underwent combined revascularization surgery. Postoperative development of the superficial temporal artery (STA), middle meningeal artery, and deep temporal artery (DTA) was assessed using the caliber-change ratio determined from magnetic resonance angiography measurements. We analyzed RNF213 and 36 other moyamoya angiopathy-related genes by whole-exome sequencing and extracted rare or damaging variants. Thirty-five participants carried RNF213 p.Arg4810Lys (all heterozygotes), whereas 5 had RNF213 rare variants (RVs). p.Arg4810Lys was significantly associated with postoperative DTA development, while age at surgery, hypertension, and hyperlipidemia were inversely associated. Multiple regression analysis revealed that age and p.Arg4810Lys held statistical significance (P = 0.044, coefficient − 0.015, 95% confidence interval (CI) − 0.029 to 0.000 and P = 0.001, coefficient 0.670, 95% CI 0.269 to 1.072, respectively). Those with RNF213 RV without p.Arg4810Lys exhibited a significant trend toward poor DTA development (P = 0.001). Hypertension demonstrated a significant positive association with STA development, which remained significant even after multiple regression analysis (P = 0.001, coefficient 0.303, 95% CI 0.123 to 0.482). Following Bonferroni correction for multiple comparisons, targeted analyses of RNF213 and 36 moyamoya angiopathy-related genes showed a significant association of only RNF213 p.Arg4810Lys with favorable DTA development (P = 0.001). A comprehensive analysis of RNF213, considering both p.Arg4810Lys and RVs, may provide a clearer prediction of postoperative DTA development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Moyamoya disease (MMD) is characterized by progressive stenosis at the terminal portion of the internal carotid artery and the development of collateral vessels at the base of the brain and other collateral networks, such as the leptomeningeal anastomosis and anastomoses between the branches of the external carotid artery and intracranial arteries [1, 2]. Another significant feature of MMD is the development of prominent synangiosis between the pial arteries and muscles, dura mater, and pericranium following surgical revascularization [1]. Owing to this characteristic, diverse variations of indirect revascularization surgery (e.g., encephalo-duro-arterio-synangiosis, and encephalo-duro-arterio-myo-synangiosis) have been considered effective for MMD and employed either as standalone procedures or in conjunction with the direct bypass (called “combined bypass”) [1, 3]. We mainly adopt a combined bypass approach in our practice, as per a recent study that demonstrated the efficacy of the combined bypass approach for facilitating the postoperative development of both direct and indirect collateral networks [4]. However, a detailed understanding of the factors contributing to the development of both direct bypass (blood flow from superficial temporal artery (STA)) and indirect bypass (blood flow from middle meningeal artery (MMA) and deep temporal artery (DTA)) could lead to the selection of more optimal surgical strategies.
Age is widely recognized as a contributory factor in postoperative bypass development in patients with MMD, as neovascularization routinely develops more robustly in younger patients, whereas adults often show suboptimal development of neovascularization [5,6,7]. Despite the reported associations with other preoperative factors, such as hyperlipidemia, disease stage, and hemorrhagic onset, there is no established consensus on these relationships [5, 8, 9].
Regarding genetic factors, RNF213 c.14429G > A (p.Arg4810Lys) is a significant risk variant for MMD [10, 11]. Controversy exists as several recent studies have reported a significant association between p.Arg4810Lys and postoperative bypass development [5, 8, 12], whereas another report has reported no such association [13]. Reports on the association between postoperative bypass development and genetic variants are limited, and to date, only associations with p.Arg4810Lys have been analyzed. A recent report indicated the possibility that RNF213 rare variants (RV) other than p.Arg4810Lys may act as modifying factors in the clinical phenotype [14]. Additionally, several other genes, known as moyamoya angiopathy-related genes, constitute potential genetic factors that contribute to the vascular phenotype characteristics of MMD or moyamoya syndrome [15, 16]. Considering the literature, these RNF213 RVs and the other genes could also be associated with postoperative bypass development. Therefore, we conducted targeted gene analysis of RNF213 and other moyamoya angiopathy-related genes to elucidate the genetic factors associated with bypass development after combined bypass surgery.
Methods
Participants
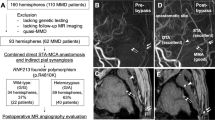
In this retrospective cohort study, we recruited patients with MMD who agreed to undergo genetic analysis and combined bypass surgery, using both direct and indirect bypass methods, at our institution between October 2011 and December 2022. This study was approved by the research board of our institution (approval number: G10026; approval date: September 12, 2011). Written informed consent was obtained from all participants or the legal guardians of patients aged < 18 years. MMD was diagnosed based on the latest criteria of the Research Committee on Moyamoya Disease [17]. For the surgical procedure, a combined bypass of the direct STA–middle cerebral artery (MCA) anastomosis with encephalo-myo-synangiosis (EMS) was performed as previously reported [18], with some minor modifications. Cases exhibiting substantial contiguous brain damage in the frontotemporal craniotomy region before surgery were excluded from bypass procedures. The reporting of this study followed STROBE guidelines (Online Resources).
Clinical and Imaging Data Collection
The clinical data shown in Table 1 were collected (for the diagnostic criteria of the medical histories, see Online Resources). Hemorrhagic symptoms included intracerebral hemorrhage, intraventricular hemorrhage, and subarachnoid hemorrhage. For cerebrovascular characteristics, we assessed time-of-flight magnetic resonance angiography (MRA) images, including Suzuki grade and PCA involvement, which was defined as a > 50% occlusion or stenosis in segments P1–P3. In cases where preoperative single-photon emission computed tomography (SPECT) imaging was used to assess the quantitative values of regional cerebral blood flow (rCBF), the ratios of the rCBF in the MCA territory to the ipsilateral cerebellum were evaluated.
Evaluation of the Postoperative Development of Direct and Indirect Collaterals
Postoperative donor artery caliber changes were assessed with MRA source images, which has been reported to correlate with postoperative bypass flow development observed on digital subtraction angiography (DSA) [19]. The development of STA reflects the development of direct bypass, while the development of MMA and DTA reflects the development of indirect bypass. The calibers of STA, MMA, and DTA were measured both pre- and postoperatively, using the method reported by Uchino et al. (Fig. S1) [19]. Then, caliber-change ratios (CCR) for each artery were calculated by dividing the postoperative caliber by the preoperative caliber. For the postoperative evaluations, we primarily used imaging performed between 6 months and 1 year after surgery. However, in cases where images from this period were unavailable for various reasons, the subsequently available images were used. Measurements of vessel calibers were assessed by two experienced neurosurgeons (T.S. and O.S.), who were blinded to the genotyping results and clinical information, and the mean values of the vessel calibers measured separately were adopted.
Whole-Exome Sequencing (WES) and Targeted Gene Analysis
WES was conducted for all participants, and Twist Comprehensive Exome Panel Kit (Twist Bioscience, South San Francisco, CA, USA) was used. Sequencing data were generated using NovaSeq6000 (Illumina, San Diego, CA, USA) and a 150 bp paired-end sequencing protocol across rapid-flow cell lanes. We confirmed that the quality was not judged as “fail” in the FastQC. Alignment to the human reference genome (Genome Reference Consortium Human Build 38 (GRCh38) [hg38]) and variant detection were performed using Clara Parabricks 3.8.0 implementation of the Burrows–Wheeler Aligner and HaplotypeCaller, respectively. Variants that passed and were annotated as “PASS” in the VCF file were analyzed. We extracted variants present in the target genes, including RNF213 and 36 other genes (previously reported as syndromic or primary moyamoya angiopathy-related genes; Table S1).
For each extracted variant, the allele frequency was analyzed using the Genome Aggregation Database (gnomAD) based on the entries in dbSNP (https://www.ncbi.nlm.nih.gov/snp/), and variant annotation was performed using ANNOVAR (https://annovar.openbioinformatics.org/en/latest/, updated on Mar 15, 2023). The variant was defined as “rare” when its minor allele frequency was < 0.01 in gnomAD. It was defined as “damaging” only if it met both of the following criteria simultaneously: (1) its combined annotation-dependent depletion score (GRCh38-v1.6) was > 20 and (2) it was categorized as “deleterious/damaging or probably deleterious/damaging” by two or more of the following four prediction tools: Sorting intolerance from Tolerant (SIFT), PolyPhen-2, MutationTaster, and Protein Variation Effect Analyzer (PROVEAN). We extracted variants that met the above criteria for being either “rare variants (RV)” or “damaging variants (DV).”
Statistical Analysis
All statistical analyses were performed using SPSS version 26 (IBM, Armonk, NY, USA). The Mann–Whitney U test was used to compare the medians between two independent groups for continuous data. The chi-square or Fisher’s exact test was used to compare the proportions of categorical variables. Linear regression analysis was applied when both the independent and dependent variables were continuous. Multiple regression analysis was used for the multivariate analysis of continuous outcomes. Statistical significance was defined at P < 0.05.
Results
Among the 63 participants (79 operated hemispheres), 41 (65.1%) were female, 35 (55.6%) were p.Arg4810Lys heterozygotes (GA), 28 (44.4%) were wild type (GG), and none were homozygotes. As regards RNF213 RV, five RVs were detected in five patients (7.9%), all of whom were GG of p.Arg4810Lys; no non-rare DVs were identified (Table S2). The RVs were p.Gly2440Asp, p.Asp2554Glu, p.Arg2704Gln, p.Met3666Thr, and p.Glu4950Asp (two of which fulfilled the current criteria for “damaging”: p.Arg2704Gln and p.Met3666Thr; Table S3). We analyzed 79 hemispheres that underwent combined bypass surgery, and their basic characteristics are summarized in Table 1. The median age of the patients at the time of surgery was 41 years. Preoperative hemispheric symptoms included transient ischemic attacks (TIA) in 44 patients (55.7%), cerebral infarctions in 24 (30.4%), and hemorrhages in 11 (13.9%). The mean duration from surgery to postoperative donor artery evaluation was 270 days. Regarding long-term postoperative clinical outcomes, there were no instances of patients experiencing an ipsilateral recurrent stroke, except for one case in which a patient suffered a symptomatic infarction in the ipsilateral frontal lobe one year after surgery.
Analysis of Factors Associated with Postoperative Development of Collaterals
We calculated the CCR of each donor artery and analyzed the significant factors associated with the CCR (Table 2). We investigated individual underlying factors, such as sex, age at surgery, medical history, smoking history, family history of MMD, and p.Arg4810Lys. Hemispheric factors included PCA involvement, Suzuki grade, hemispheric preoperative symptoms, and the reciprocal relationship between the postoperative development of direct and indirect bypass.
Hypertension was significantly associated with the CCR of STA (P = 0.007). Regarding the CCR of DTA, p.Arg4810Lys showed a positive association (P = 0.001), whereas age at surgery, hypertension, and hyperlipidemia were negatively associated (P = 0.001, 0.007, and 0.027, respectively; associations of hypertension with the CCR of STA and DTA are shown in Fig. 1). No factor was associated with the CCR of MMA. Regarding the relationship between the CCR of STA and hypertension, even after adjustment (multiple regression analysis) for age at surgery, hypertension remained statistically significant (P = 0.001, coefficient 0.303, 95% confidence interval (CI) 0.123 to 0.482; Table 3). Following the univariate analyses for the CCR of DTA, a subsequent multiple regression analysis revealed that only age at surgery and p.Arg4810Lys retained statistical significance (P = 0.044, coefficient − 0.015, 95% CI − 0.029 to 0.000 and P = 0.001, coefficient 0.670, 95% CI 0.269 to 1.072, respectively; Table 3 and Fig. 2a, 2b). Meanwhile, there was no significant association between the preoperative Suzuki grade and preoperative symptoms with the CCR of any vessel (Table 2). Furthermore, although the analysis was limited to cases for which SPECT data were available, the preoperative rCBF reduction in the MCA territory was not significantly associated with the genotype of RNF213 or with the bypass development (Tables S4 and S5). These findings suggest that the outcomes of our study were not significantly influenced by preoperative hemodynamic compromise.
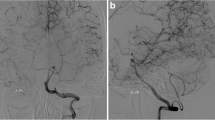
Effect of hypertension on the caliber-change ratio (CCR) of DTA and STA. The association between the CCR of STA and DTA and hypertension is shown in the boxplots (a, b). The CCR of STA is higher in patients with hypertension, whereas CCR of DTA is lower in those with hypertension; both with a P-value of 0.007. Images (c–f) show the preoperative and postoperative MRA of representative cases: one with hypertension (c, d) and another without hypertension (e, f). These two patients were of comparable age, female, without RNF213 p.Arg4810Lys, and both presented with preoperative infarctions. In image d, the STA (indicated by an arrow) is well-developed, whereas the DTA is scarcely developed. In image f, the STA (indicated by an arrow) is patent but underdeveloped, whereas the DTA (indicated by an arrowhead) shows good development. The P-value was calculated using the Mann–Whitney U test. *P < 0.01. GG, wild type of p.Arg4810Lys; HT, hypertension
Statistically significant risk factors for the caliber-change ratio (CCR) of DTA, and comparison among genotypes of RNF213. a A scatter plot and regression analysis result showing the relationship between age at surgery and the CCR of DTA. Red dots represent the cases with GA, while blue dots indicate those with GG. P-values and regression coefficients were determined by regression analysis. Pearson’s correlation coefficient was − 0.353. “B” represents the unstandardized regression coefficient, which was − 0.022 (95% confidence interval − 0.035 to − 0.009). b A boxplot illustrating the association between the CCR of DTA and RNF213 p.Arg4810Lys. P-value were computed using the Mann–Whitney U test. *P < 0.005. c A boxplot comparing the CCR of DTA among three genotype groups: GG/RV − , GG/RV + , and GA. P-values were determined using the Kruskal–Wallis test. *P < 0.005, **P < 0.05 followed by Bonferroni correction. GA, heterozygote of p.Arg4810Lys; GG, wild type of p.Arg4810Lys; RV, rare variant
Association of RV and DV with Postoperative Collaterals: Targeted Analysis on RNF213 and Other Moyamoya Angiopathy-Related Genes
RVs and DVs were extracted from the WES results based on the earlier definitions and focusing on RNF213 as well as the other 36 moyamoya angiopathy-related genes.
For RNF213, five RVs were identified in patients with GG, as previously described. A boxplot charting the CCR of DTA across the GG/RV − , GG/RV + , and GA groups revealed significant differences between the GA and the GG/RV + group (P = 0.001, corrected significance level followed by Bonferroni correction: P < 0.017; Fig. 2c).
The moyamoya angiopathy-related genes ACTA2, PTPN1, SOS1, HRAS, SMARCAL1, CECR1, SAMHD1, HBB, and EVL did not have RVs or DVs. Among the remaining 27 moyamoya angiopathy-related genes, we identified 11 genes that exhibited either RV or DV in more than five hemispheres (for information on the variants detected in these 11 genes, see Table S6). The Mann–Whitney U test was used to determine whether the presence of RNF213 p.Arg4810Lys, RNF213 RVs, or these 11 genes was associated with the CCR of each vessel. Intergroup differences in the median CCR in groups with and without variants were visualized using a heatmap (Fig. 3). Following Bonferroni correction for multiple comparisons in each vessel, no statistically significant genes remained in STA or MMA. However, as for DTA, RNF213 p.Arg4810Lys was significantly associated with an increased CCR (P = 0.001, corrected significance level followed by Bonferroni correction: P < 0.0038). RNF213 RVs demonstrated a more pronounced negative association with the CCR of DTA than with any other gene, although they did not reach significance after correction (P = 0.004).
Heatmap representing the association of genetic variants with the caliber-change ratio of STA, MMA, and DTA. Values represent the differences in median CCR of each vessel between the groups with and without variants. * indicates statistical significance, even after Bonferroni correction (corrected significance level: P < 0.0038)
Discussion
In this study, we performed WES and targeted analyses for RNF213 as well as previously reported moyamoya angiopathy-related genes. The current findings indicate a pronounced association between RNF213 and the postoperative development of DTA after combined bypass surgery, suggesting that p.Arg4810Lys can enhance DTA development and that RNF213 RVs may reduce it. Furthermore, we found that younger age at surgery was associated with better DTA development. We also found that hypertension could be associated with altered vascular development, characterized by reduced DTA development and enhanced STA development.
Based on the current results, optimal surgical approaches can be determined before surgery. First, younger patients can be expected to have better DTA development after EMS. Even in older patients, p.Arg4810Lys still facilitates DTA development, indicating that combining EMS with direct bypass could be a more effective surgical approach in patients with this variant. Secondly, although DTA development tends to be poor in patients with hypertension, STA development may be significantly better. Thus, an emphasis on direct bypass over indirect bypass can be warranted in patients with hypertension.
p.Arg4810Lys is associated with certain MMD phenotypes. Carriers of this variant are more likely to exhibit PCA involvement [20, 21] and be more susceptible to cerebral ischemic symptoms [21,22,23]. Additionally, several reports have suggested the association of p.Arg4810Lys with the development of non-operative or postoperative collaterals. Ge et al. reported significant postoperative bypass development in p.Arg4810Lys carriers across cohorts including direct, indirect, and combined bypass surgeries [5]. Similarly, Ito et al. and Kawabori et al. reported enhanced development of indirect bypass from DTA in both adult and pediatric MMD patients with p.Arg4810Lys [8, 12]. In contrast to these reports, Zheng et al. asserted that, in a cohort of pediatric patients with MMD who underwent indirect bypass, there was no significant association between p.Arg4810Lys and postoperative bypass development [13]. In the context of non-operative collaterals, Kim et al. reported, albeit in a small cohort, that compared to those without the variant, adult MMD patients with p.Arg4810Lys exhibited poorer development of leptomeningeal collaterals [24]. Moreover, Ge et al. noted that while p.Arg4810Lys carriers exhibit poor development of the leptomeningeal collateral network, they demonstrate robust collateral development from branches of the external carotid artery [25]. By integrating these previous findings and the result of this study, it can be inferred that, due to poor leptomeningeal collateral development, p.Arg4810Lys carriers might develop enhanced bypass flow to compensate for ischemic conditions. In support of this hypothesis, animal studies have shown enhanced angiogenesis following hindlimb ischemia in RNF213 knockout mice [26]. Although RNF213 knockout mouse models have not shown significant cerebrovascular anomalies like MMD, and the pathophysiological mechanisms by which RNF213 affects MMD remain unclear [16], further research is needed in the future to elucidate this intriguing relation between p.Arg4810Lys and enhanced angiogenesis.
In addition to p.Arg4810Lys, associations of RNF213 RVs with certain MMD phenotypes have been reported [27,28,29]. Hara et al. reported that in pediatric-onset MMD undergoing indirect bypass surgery, patients without p.Arg4810Lys exhibited poorer postoperative outcomes compared to those with p.Arg4810Lys. Moreover, these p.Arg4810Lys-negative patients frequently had RNF213 RVs (eight of 25 patients) [28]. Our finding of a potential relationship between RNF213 RVs and poor postoperative DTA development may provide an explanation for their results. Notably, most of the RNF213 RVs (four of five RVs) we identified in our cohort demonstrated higher CADD scores than p.Arg4810Lys, suggesting more severe functional effects on the RNF213 protein. This study is the first to suggest that RNF213 variants, other than p.Arg4810Lys, may be associated with bypass development. Despite the preliminary nature of our findings, they raise the possibility that sequencing all variants of RNF213 may offer predictive value for postoperative indirect bypass development.
Furthermore, we analyzed potential genes other than RNF213 that were related to moyamoya angiopathy. To the best of our knowledge, this is the first study to investigate the association between postoperative bypass development and the variants of genes other than RNF213. Besides RNF213, no other gene was significantly associated with postoperative donor artery development. The moyamoya angiopathy-related genes we analyzed have been implicated in processes such as vascular remodeling, actin remodeling, the cell cycle, and the mitogen-activated protein kinase (MAPK) pathway [15, 16]. Thus, they have potential roles in vascular phenotypes. For instance, abnormal angiogenesis has been observed in BRCC3-knockdown zebrafish [30], and recent findings suggest that RVs in ANO1 may impact cell membrane potential, thereby potentially influencing the function of vascular smooth muscle and endothelial cells [31]. Although analyses focused on RNF213 p.Arg4810Lys have been the mainstream approach in MMD, it seems increasingly important to accumulate comprehensive phenotype–genotype analyses, considering the potential implications of these moyamoya angiopathy-related genes.
Regarding the association with patient characteristics other than genetic factors, two significant findings were evident from the multivariate analysis in this study: younger age and hypertension were associated with better DTA and STA development, respectively. The associations of Suzuki grade or hemorrhagic onset with postoperative collateral development, as previously reported [4, 5], were not confirmed in our study. The association between age and the development of indirect bypass was consistent with previous reports [5,6,7]. Few reports have analyzed the relationship between hypertension and postoperative bypass development. Among them, Ito et al. uniquely analyzed postoperative direct or indirect bypass separately in a cohort with combined bypass; however, no significant association with hypertension was demonstrated [8]. Although historical analyses have often underrepresented the association between the development of STA after combined bypass and hypertension, it is important to emphasize the need for further studies. By establishing a clearer relationship between postoperative bypass development and factors such as age or hypertension, we can make considerable contributions to determining more optimal surgical strategies.
This study had several limitations. First, this was a retrospective cohort study, which inherently introduced a selection bias, and the sample size was relatively small. We focused only on patients in whom WES could be performed and did not register cases continuously. This study design likely contributed to the comparatively low proportion of RNF213 p.Arg4810Lys heterozygotes observed in our study, as compared to the higher frequencies reported in the existing literature. Therefore, it is crucial to interpret our findings with an understanding of this limitation, as it may influence the generalizability and applicability of our results. Second, we did not analyze genome-wide genes other than RNF213. Our analysis was limited to genes that were previously reported to be associated with moyamoya angiopathy. Third, our evaluation was based solely on the changes in the diameter of donor arteries. We did not analyze blood-flow distribution on DSA or perform hemodynamic evaluation on SPECT because these procedures were not performed in all cases. Additionally, it is important to note that our analysis did not include detailed postoperative outcomes. These outcomes include asymptomatic minor strokes that may have been incidentally detected on follow-up MRI, as well as subjective symptoms that are not apparent in follow-up imaging. The only exception to this is the single case that suffered from a symptomatic recurrent infarction, which is described in the Results section. Lastly, the exact pathophysiological mechanism by which RNF213 variants influence donor artery development remains unknown, necessitating further functional analyses to elucidate this pathology in future studies.
Conclusion
In targeted gene analysis, including previously reported moyamoya angiopathy-related genes, RNF213 was identified as the most influential gene related to postoperative DTA development. p.Arg4810Lys is significantly associated with enhanced DTA development, whereas RNF213 RVs may exert inhibitory effects on DTA development. The profiling of RNF213 p.Arg4810Lys and RVs can enable the stratification and prediction of the outcomes of indirect revascularization procedures; thus, a comprehensive genetic analysis of RNF213 is crucial.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request from any investigator.
Abbreviations
- DTA:
-
Deep temporal artery
- GA:
-
Heterozygote of p.Arg4810Lys
- GG:
-
Wild type of p.Arg4810Lys
- IQR:
-
Interquartile range
- MMA:
-
Middle meningeal artery
- MMD:
-
Moyamoya disease
- NA:
-
Not applicable
- rCBF:
-
Regional cerebral blood flow
- SPECT:
-
Single-photon emission computed tomography
- STA:
-
Superficial temporal artery
- TIA:
-
Transient ischemic attack
- WES:
-
Whole-exome sequencing
References
Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008;7:1056–66. https://doi.org/10.1016/S1474-4422(08)70240-0.
Scott RM, Smith ER. Moyamoya disease and Moyamoya syndrome. N Engl J Med. 2009;360:1226–37. https://doi.org/10.1056/NEJMra0804622.
Houkin K, Kuroda S, Ishikawa T, Abe H. Neovascularization (angiogenesis) after revascularization in Moyamoya disease. Which technique is most useful for moyamoya disease? Acta Neurochir. 2000;142:269–76. https://doi.org/10.1007/s007010050035.
Uchino H, Kim JH, Fujima N, Kazumata K, Ito M, Nakayama N, Kuroda S, Houkin K. Synergistic interactions between direct and indirect bypasses in combined procedures: the significance of indirect bypasses in Moyamoya disease. Neurosurgery. 2017;80:201–9. https://doi.org/10.1227/NEU.0000000000001201.
Ge P, Ye X, Liu X, Deng X, Wang J, Wang R, Zhang Y, Zhang D, Zhang Q, Zhao J. Association between p.R4810K variant and postoperative collateral formation in patients with moyamoya disease. Cerebrovasc Dis. 2019;48:77–84. https://doi.org/10.1159/000503250.
Czabanka M, Vajkoczy P, Schmiedek P, Horn P. Age-dependent revascularization patterns in the treatment of moyamoya disease in a European patient population. Neurosurg Focus. 2009;26:E9. https://doi.org/10.3171/2009.1.FOCUS08298.
Lee SB, Kim DS, Huh PW, Yoo DS, Lee TG, Cho KS. Long-term follow-up results in 142 adult patients with moyamoya disease according to management modality. Acta Neurochir. 2012;154:1179–87. https://doi.org/10.1007/s00701-012-1325-1.
Ito M, Kawabori M, Sugiyama T, Tokairin K, Tatezawa R, Uchino H, Kazumata K, Houkin K, Fujimura M. Impact of RNF213 founder polymorphism (p.R4810K) on the postoperative development of indirect pial synangiosis after direct/indirect combined revascularization surgery for adult Moyamoya disease. Neurosurg Rev. 2022;45:2305–13. https://doi.org/10.1007/s10143-022-01749-9.
Zhao Y, Li J, Lu J, Zhang Q, Zhang D, Wang R, Zhao Y, Chen X. Predictors of neoangiogenesis after indirect revascularization in moyamoya disease: a multicenter retrospective study. J Neurol Surg. 2020;132:98–108. https://doi.org/10.3171/2018.9.jns181562.
Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A, Kanno J, Niihori T, Ono M, Ishii N, Owada Y, Fujimura M, Mashimo Y, Suzuki Y, Hata A, Tsuchiya S, Tominaga T, Matsubara Y, Kure S. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet. 2011;56:34–40. https://doi.org/10.1038/jhg.2010.132.
Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T, Hashikata H, Matsuura N, Yamazaki S, Toyoda A, Kikuta K, Takagi Y, Harada KH, Fujiyama A, Herzig R, Krischek B, Zou L, Kim JE, Kitakaze M, Miyamoto S, Nagata K, Hashimoto N, Koizumi A. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS ONE. 2011;6:e22542. https://doi.org/10.1371/journal.pone.0022542.
Kawabori M, Ito M, Kazumata K, Tokairin K, Hatanaka KC, Ishikawa S, Houkin K, Fujimuraet M. Impact of RNF213 c.14576G>A Variant on the development of direct and indirect revascularization in pediatric moyamoya disease. Cerebrovasc Dis. 2023;52:171–6. https://doi.org/10.1159/000526089.
Zheng EY, Hara S, Inaji M, Tanaka Y, Nariai T, Maehara T. Regression of periventricular anastomosis after indirect revascularization in pediatric patients with moyamoya disease. J Neurosurg Pediatr. 2023;1–10. https://doi.org/10.3171/2023.8.PEDS23304.
Torazawa S, Miyawaki S, Imai H, Hongo H, Ishigami D, Shimizu M, Ono H, Shinya Y, Sato D, Sakai Y, Umekawa M, Kiyofuji S, Shimada D, Koizumi S, Komura D, Katoh H, Ishikawa S, Nakatomi H, Teraoka A, Saito N. RNF213 p.Arg4810Lys wild type is associated with de novo hemorrhage in asymptomatic hemispheres with moyamoya disease. Transl Stroke Res. 2023;Arg4810Lys. https://doi.org/10.1007/s12975-023-01159-z.
Guey S, Tournier-Lasserve E, Hervé D, Kossorotoff M. Moyamoya disease and syndromes: from genetics to clinical management. Appl Clin Genet. 2015;8:49–68. https://doi.org/10.2147/TACG.S42772.
Ihara M, Yamamoto Y, Hattori Y, Liu W, Kobayashi H, Ishiyama H, Yoshimoto T, Miyawaki S, Clausen T, Bang OY, Steinberg GK, Tournier-Lasserve E, Koizumi A. Moyamoya disease: diagnosis and interventions. Lancet Neurol. 2022;21:747–58. https://doi.org/10.1016/S1474-4422(22)00165-X.
Kuroda S, Fujimura M, Takahashi J, Kataoka H, Ogasawara K, Iwama T, Tominaga T, Miyamoto S. Diagnostic criteria for moyamoya disease – 2021 revised version. Neurol Med Chir (Tokyo). rev. version. 2022;62:307–12. https://doi.org/10.2176/jns-nmc.2022-0072.
Imai H, Miyawaki S, Ono H, Nakatomi H, Yoshimoto Y, Saito N. The importance of encephalo-myo-synangiosis in surgical revascularization strategies for Moyamoya disease in children and adults. World Neurosurg. 2015;83:691–9. https://doi.org/10.1016/j.wneu.2015.01.016.
Uchino H, Yamamoto S, Kashiwazaki D, Akioka N, Kuwayama N, Noguchi K, Kuroda S. Using postoperative remodeling of donor arteries on MR angiography to predict the development of surgical collaterals in Moyamoya disease. J Neurosurg. 2019;1–9. https://doi.org/10.3171/2019.8.JNS191846.
Wang Y, Zhang Z, Wang X, Zou Z, Ta N, Hao F, Yang Y, Li D, Liang M, Han C, Bao X, Ou L, Wang H, Yang Z, Yang R, Zeng F, Shang M, Nie F, Liu W, Duan L. Validation and extension study exploring the role of RNF213 p.R4810K in 2,877 Chinese moyamoya disease patients. J Stroke Cerebrovasc Dis. 2021;30:106071. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106071.
Ge P, Ye X, Liu X, Deng X, Wang R, Zhang Y, Zhang D, Zhang Q, Zhao J. Association between p.R4810K variant and long-term clinical outcome in patients with moyamoya disease. Front Neurol. 2019;10:662. https://doi.org/10.3389/fneur.2019.00662.
Nomura S, Yamaguchi K, Akagawa H, Kawashima A, Moteki Y, Ishikawa T, Aihara Y, Saito T, Okada Y, Kawamata T. Genotype-phenotype correlation in long-term cohort of Japanese patients with Moyamoya disease. Cerebrovasc Dis. 2019;47:105–11. https://doi.org/10.1159/000499699.
Wu Z, Jiang H, Zhang L, Xu X, Zhang X, Kang Z, Song D, Zhang J, Guan M, Gu Y. Molecular analysis of RNF213 gene for moyamoya disease in the Chinese Han population. PLOS ONE. 2012;7:e48179. https://doi.org/10.1371/journal.pone.0048179.
Kim WH, Kim SD, Nam MH, Jung JM, Jin SW, Ha SK, Lim D-J, Lee H-B. Posterior circulation involvement and collateral flow pattern in moyamoya disease with the RNF213 polymorphism. Childs Nerv Syst. 2019;35:309–14. https://doi.org/10.1007/s00381-018-3985-5.
Ge P, Zhang Q, Ye X, Liu X, Deng X, Wang J, Wang R, Zhang Y, Zhang D, Zhao J. Angiographic characteristics in moyamoya disease with the p.R4810K variant: a propensity score-matched analysis variant: a propensity score-matched analysis. Eur J Neurol. 2020;27:856–63. https://doi.org/10.1111/ene.14184.
Ito A, Fujimura M, Niizuma K, Kanoke A, Sakata H, Morita-Fujimura Y, Kikuchi A, Kure S, Tominaga T. Enhanced post-ischemic angiogenesis in mice lacking RNF213; a susceptibility gene for moyamoya disease. Brain Res. 2015;1594:310–20. https://doi.org/10.1016/j.brainres.2014.11.014.
Guey S, Kraemer M, Hervé D, Ludwig T, Kossorotoff M, Bergametti F, Schwitalla JC, Choi S, Broseus L, Callebaut I, Genin E, Tournier-Lasserve E, FREX consortium. Rare RNF213 variants in the C-terminal region encompassing the RING-finger domain are associated with moyamoya angiopathy in Caucasians. Eur J Hum Genet. 2017;25:995–1003. https://doi.org/10.1038/ejhg.2017.92.
Hara S, Mukawa M, Akagawa H, Thamamongood T, Inaji M, Tanaka Y, Maehara T, Kasuya H, Nariai T. Absence of the RNF213 p.R4810K variant may indicate a severe form of pediatric moyamoya disease in Japanese patients. J Neurosurg Pediatr. 2022;29:48–56. https://doi.org/10.3171/2021.7.PEDS21250.
Nomura S, Akagawa H, Yamaguchi K, Azuma K, Nakamura A, Fukui A, Matsuzawa F, Aihara Y, Ishikawa T, Moteki Y, Chiba K, Hashimoto K, Morita S, Ishiguro T, Okada Y, Vetiska S, Andrade-Barazarte H, Radovanovic I, Kawashima A, Kawamata T. Difference in clinical phenotype, mutation position, and structural change of RNF213 rare variants between pediatric and adult Japanese patients with Moyamoya disease. Transl Stroke Res. 2023. https://doi.org/10.1007/s12975-023-01194-w.
Miskinyte S, Butler MG, Hervé D, Sarret C, Nicolino M, Petralia JD, Bergametti F, Arnould M, Pham VN, Gore AV, Spengos K, Gazal S, Woimant F, Steinberg GK, Weinstein BM, Tournier-Lasserve E. Loss of BRCC3 deubiquitinating enzyme leads to abnormal angiogenesis and is associated with syndromic moyamoya. Am J Hum Genet. 2011;88:718–28. https://doi.org/10.1016/j.ajhg.2011.04.017.
Pinard A, Ye W, Fraser SM, Rosenfeld JA, Pichurin P, Hickey SE, Guo D, Cecchi AC, Boerio ML, Guey S, Aloui C, Lee K, Kraemer M, Alyemni SO; University of Washington Center for Mendelian Genomics; Bamshad MJ, Nickerson DA, Tournier-Lasserve E, Haider S, Jin SC, Smith ER, Kahle KT, Jan LY, He M, Milewicz DM. Rare variants in ANO1, encoding a calcium-activated chloride channel, predispose to moyamoya disease. Brain. 2023;146:3616–23. https://doi.org/10.1093/brain/awad172.
Acknowledgements
We express our sincere gratitude to Yosuke Inaba and Kosuke Kashiwabara for their invaluable advice regarding the statistical analyses presented in this study.
Funding
Open Access funding provided by The University of Tokyo. This work was supported by Japan Society for the Promotion of Science KAKENHI (grant no. 21H03041 to Dr. Saito, 23H03018 to Dr. Miyawaki, and 23KJ0446 to Dr. Torazawa). This study was also supported by grants from the Charitable Trust Mihara Cerebrovascular Disorder Research Promotion Fund to Dr. Miyawaki and grants from the MSD Life Science Foundation (Public Interest Incorporated Foundation) to Dr. Hongo.
Author information
Authors and Affiliations
Contributions
S.M. and N.S. supervised the study. S.T. and S.M. wrote the manuscript. S.T., D.K., H.K., and S.I. conducted data analysis. S.T., S.M., H.I., H.H., H.O., S.O., Y.S., S.K., and S.K. collected the samples. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the research board of our institution (approval number: G10026; approval date: September 12, 2011). This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to Participate
Written informed consent was obtained from all participants or the legal guardians of patients aged < 18 years.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torazawa, S., Miyawaki, S., Imai, H. et al. Association of Genetic Variants with Postoperative Donor Artery Development in Moyamoya Disease: RNF213 and Other Moyamoya Angiopathy-Related Gene Analysis. Transl. Stroke Res. (2024). https://doi.org/10.1007/s12975-024-01248-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12975-024-01248-7