Abstract
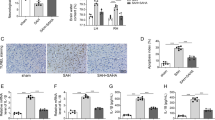
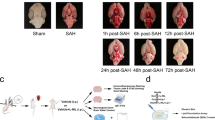
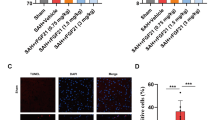
M1 microglial activation is crucial for the pathogenesis of early brain injury (EBI) following subarachnoid hemorrhage (SAH), and there is growing evidence that glucose metabolism is frequently involved in microglial activation. However, the molecular mechanism of glycolysis and its role in M1 microglial activation in the context of EBI are not yet fully understood. In this study, firstly, the relationship between aerobic glycolysis and M1 microglial activation as well as SAH-induced EBI was researched in vivo. Then, intervention on mammalian target of rapamycin (mTOR) was performed to investigate the effects on glycolysis-dependent M1 microglial activation and EBI and its relationship with hypoxia-inducible factor-1α (HIF-1α) in vivo. Next, Hif-1α was inhibited to analyze its role in aerobic glycolysis, M1 microglial activation, and EBI in vivo. Lastly, both in vivo and in vitro, mTOR inhibition and Hif-1α enhancement were administered simultaneously, and the combined effects were further confirmed again. The results showed that aerobic glycolysis and M1 microglial polarization were increased after SAH, and glycolytic inhibition could attenuate M1 microglial activation and EBI. Inhibition of mTOR reduced glycolysis-dependent M1 microglial polarization and EBI severity by down-regulating HIF-1α expression, while enhancement had the opposite effects. Blockading HIF-1α had the similar effects as suppressing mTOR, while HIF-1α agonist worked against mTOR antagonist when administered simultaneously. In conclusion, the present study showed new evidence that aerobic glycolysis induced by mTOR/HIF-1α might promote EBI after SAH by activating M1 microglia. This finding provided new insights for the treatment of EBI.









Similar content being viewed by others
Data Availability
The authors confirm that the findings of this study are supported by the data therein. The data are available from the corresponding author.
References
Muehlschlegel S. Subarachnoid hemorrhage. Continuum (Minneap Minn). 2018;24(6):1623–57. https://doi.org/10.1212/CON.0000000000000679.
Rass V, Helbok R. Early brain injury after poor-grade subarachnoid hemorrhage. Curr Neurol Neurosci Rep. 2019;19(10):78. https://doi.org/10.1007/s11910-019-0990-3.
Xu G, Guo J, Sun C. Eucalyptol ameliorates early brain injury after subarachnoid haemorrhage via antioxidant and anti-inflammatory effects in a rat model. Pharm Biol. 2021;59(1):114–20. https://doi.org/10.1080/13880209.2021.1876101.
Xu W, Mo J, Ocak U, Travis ZD, Enkhjargal B, Zhang T, et al. Activation of melanocortin 1 receptor attenuates early brain injury in a rat model of subarachnoid hemorrhage viathe suppression of neuroinflammation through AMPK/TBK1/NF-kappaB pathway in rats. Neurotherapeutics. 2020;17(1):294–308. https://doi.org/10.1007/s13311-019-00772-x.
Yang C, Li T, Xue H, Wang L, Deng L, Xie Y, et al. Inhibition of necroptosis rescues SAH-induced synaptic impairments in hippocampus via CREB-BDNF pathway. Front Neurosci. 2018;12:990. https://doi.org/10.3389/fnins.2018.00990.
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–8. https://doi.org/10.1038/nn.3469.
Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193–201. https://doi.org/10.1038/nrneurol.2010.17.
Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11(11):775–87. https://doi.org/10.1038/nri3086.
Tanaka T, Murakami K, Bando Y, Yoshida S. Interferon regulatory factor 7 participates in the M1-like microglial polarization switch. Glia. 2015;63(4):595–610. https://doi.org/10.1002/glia.22770.
McGettrick AF, O’Neill LA. How metabolism generates signals during innate immunity and inflammation. J Biol Chem. 2013;288(32):22893–8. https://doi.org/10.1074/jbc.R113.486464.
Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173(4):649–65. https://doi.org/10.1111/bph.13139.
Cheng J, Zhang R, Xu Z, Ke Y, Sun R, Yang H, et al. Early glycolytic reprogramming controls microglial inflammatory activation. J Neuroinflammation. 2021;18(1):129. https://doi.org/10.1186/s12974-021-02187-y.
Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, et al. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39(1):126. https://doi.org/10.1186/s13046-020-01629-4.
Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25(7):771–84. https://doi.org/10.1038/cr.2015.68.
Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI, et al. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab. 2019;30(3):493-507 e496. https://doi.org/10.1016/j.cmet.2019.06.005.
You W, Wang Z, Li H, Shen H, Xu X, Jia G, et al. Inhibition of mammalian target of rapamycin attenuates early brain injury through modulating microglial polarization after experimental subarachnoid hemorrhage in rats. J Neurol Sci. 2016;367:224–31. https://doi.org/10.1016/j.jns.2016.06.021.
Sun XG, Duan H, Jing G, Wang G, Hou Y, Zhang M. Inhibition of TREM-1 attenuates early brain injury after subarachnoid hemorrhage via downregulation of p38MAPK/MMP-9 and preservation of ZO-1. Neuroscience. 2019;406:369–75. https://doi.org/10.1016/j.neuroscience.2019.03.032.
Charpentier C, Audibert G, Guillemin F, Civit T, Ducrocq X, Bracard S, et al. Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke. 1999;30(7):1402–8. https://doi.org/10.1161/01.str.30.7.1402.
Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167(2):327–34. https://doi.org/10.1016/j.jneumeth.2007.08.004.
Li Y, Wu P, Dai J, Zhang T, Bihl J, Wang C, et al. Inhibition of mTOR alleviates early brain injury after subarachnoid hemorrhage via relieving excessive mitochondrial fission. Cell Mol Neurobiol. 2020;40(4):629–42. https://doi.org/10.1007/s10571-019-00760-x.
Zhou D, Matchett GA, Jadhav V, Dach N, Zhang JH. The effect of 2-methoxyestradiol, a HIF-1 alpha inhibitor, in global cerebral ischemia in rats. Neurol Res. 2008;30(3):268–71. https://doi.org/10.1179/016164107X229920.
Nomura E, Ohta Y, Tadokoro K, Shang J, Feng T, Liu X, et al. Imaging hypoxic stress and the treatment of amyotrophic lateral sclerosis with dimethyloxalylglycine in a mice model. Neuroscience. 2019;415:31–43. https://doi.org/10.1016/j.neuroscience.2019.06.025.
Wang J, Yang C, Hou X, Xu J, Yun Y, Qin L, et al. Rapamycin modulates the proinflammatory memory-like response of microglia induced by BAFF. Front Immunol. 2021;12:639049. https://doi.org/10.3389/fimmu.2021.639049.
Li Y, Han W, Wu Y, Zhou K, Zheng Z, Wang H, et al. Stabilization of hypoxia inducible factor-1alpha by dimethyloxalylglycine promotes recovery from acute spinal cord injury by inhibiting neural apoptosis and enhancing axon regeneration. J Neurotrauma. 2019;36(24):3394–409. https://doi.org/10.1089/neu.2018.6364.
Peng Y, Zhuang J, Ying G, Zeng H, Zhou H, Cao Y, et al. Stimulator of IFN genes mediates neuroinflammatory injury by suppressing AMPK signal in experimental subarachnoid hemorrhage. J Neuroinflammation. 2020;17(1):165. https://doi.org/10.1186/s12974-020-01830-4.
Li T, Xu W, Gao L, Guan G, Zhang Z, He P, et al. Mesencephalic astrocyte-derived neurotrophic factor affords neuroprotection to early brain injury induced by subarachnoid hemorrhage via activating Akt-dependent prosurvival pathway and defending blood-brain barrier integrity. FASEB J. 2019;33(2):1727–41. https://doi.org/10.1096/fj.201800227RR.
Xu P, Zhang X, Liu Q, Xie Y, Shi X, Chen J, et al. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019;10(8):555. https://doi.org/10.1038/s41419-019-1777-9.
Duan H, Li L, Shen S, Ma Y, Yin X, Liu Z, et al. Hydrogen sulfide reduces cognitive impairment in rats after subarachnoid hemorrhage by ameliorating neuroinflammation mediated by the TLR4/NF-kappaB pathway in microglia. Front Cell Neurosci. 2020;14:210. https://doi.org/10.3389/fncel.2020.00210.
Wei S, Luo C, Yu S, Gao J, Liu C, Wei Z, et al. Erythropoietin ameliorates early brain injury after subarachnoid haemorrhage by modulating microglia polarization via the EPOR/JAK2-STAT3 pathway. Exp Cell Res. 2017;361(2):342–52. https://doi.org/10.1016/j.yexcr.2017.11.002.
Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995; 26(4): 627–634; discussion 635.https://doi.org/10.1161/01.str.26.4.627.
Xu P, Hong Y, Xie Y, Yuan K, Li J, Sun R, et al. TREM-1 exacerbates neuroinflammatory injury via NLRP3 inflammasome-mediated pyroptosis in experimental subarachnoid hemorrhage. Transl Stroke Res. 2021;12(4):643–59. https://doi.org/10.1007/s12975-020-00840-x.
Obara-Michlewska M, Ding F, Popek M, Verkhratsky A, Nedergaard M, Zielinska M, et al. Interstitial ion homeostasis and acid-base balance are maintained in oedematous brain of mice with acute toxic liver failure. Neurochem Int. 2018;118:286–91. https://doi.org/10.1016/j.neuint.2018.05.007.
McKelvey KJ, Wilson EB, Short S, Melcher AA, Biggs M, Diakos CI, et al. Glycolysis and fatty acid oxidation inhibition improves survival in glioblastoma. Front Oncol. 2021;11:633210. https://doi.org/10.3389/fonc.2021.633210.
Morishima M, Horikawa K, Funaki M. Cardiomyocytes cultured on mechanically compliant substrates, but not on conventional culture devices, exhibit prominent mitochondrial dysfunction due to reactive oxygen species and insulin resistance under high glucose. PLoS ONE. 2018;13(8):e0201891. https://doi.org/10.1371/journal.pone.0201891.
O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–55. https://doi.org/10.1038/nature11862.
Luo G, Wang X, Cui Y, Cao Y, Zhao Z, Zhang J. Metabolic reprogramming mediates hippocampal microglial M1 polarization in response to surgical trauma causing perioperative neurocognitive disorders. J Neuroinflammation. 2021;18(1):267. https://doi.org/10.1186/s12974-021-02318-5.
Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12(1):71. https://doi.org/10.1186/s13045-019-0754-1.
Hu Y, Mai W, Chen L, Cao K, Zhang B, Zhang Z, et al. mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP. Glia. 2020;68(5):1031–45. https://doi.org/10.1002/glia.23760.
Huang W, Ding X, Ye H, Wang J, Shao J, Huang T. Hypoxia enhances the migration and invasion of human glioblastoma U87 cells through PI3K/Akt/mTOR/HIF-1alpha pathway. NeuroReport. 2018;29(18):1578–85. https://doi.org/10.1097/WNR.0000000000001156.
Xia M, Ding Q, Zhang Z, Feng Q. Remote limb ischemic preconditioning protects rats against cerebral ischemia via HIF-1alpha/AMPK/HSP70 pathway. Cell Mol Neurobiol. 2017;37(6):1105–14. https://doi.org/10.1007/s10571-016-0444-2.
Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007;282(28):20534–43. https://doi.org/10.1074/jbc.M611782200.
Pawlus MR, Hu CJ. Enhanceosomes as integrators of hypoxia inducible factor (HIF) and other transcription factors in the hypoxic transcriptional response. Cell Signal. 2013;25(9):1895–903. https://doi.org/10.1016/j.cellsig.2013.05.018.
Fu M, Zhu Y, Zhang J, Wu W, Sun Y, Zhang X, et al. MicroRNA-221-3p suppresses the microglia activation and seizures by inhibiting of HIF-1alpha in valproic acid-resistant epilepsy. Front Pharmacol. 2021;12:714556. https://doi.org/10.3389/fphar.2021.714556.
Acknowledgements
We would like to thank the following projects for funding this research: Shanxi Province’s Great Research Project, Shanxi Health Committee’s Project, and Shanxi Province’s Basic Research Plan.
Funding
This study was supported by Shanxi Province’s Great Research Project (201903D321044), Shanxi Health Committee’s Project (2019045), and Shanxi Province’s Basic Research Plan (20210302123264).
Author information
Authors and Affiliations
Contributions
Xin-Gang Sun and Xue-Hong Chu designed the study and drafted the manuscript for content, including medical writing for content; Shao-Yu Liu, Yi-Bo Zhang, Li-Juan Zhu, Juan Huang, and Hai Wang had done experiments and obtained data; Ivan Steve Godje Godje, Hui-Yu Hu, Chen Sui, and Ying-Jie Shen analyzed and organized data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study was conducted in accordance with the ARRIVE guidelines and approved by the Animal and Ethics Review Committee of the Second Hospital Affiliated to Shanxi Medical University of China. All experimental procedures were conducted according to the ARRIVE guidelines. The protocols for the animal experiments were performed according to the Animal and Ethics Review Committee of our institution.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, XG., Chu, XH., Godje Godje, I.S. et al. Aerobic Glycolysis Induced by mTOR/HIF-1α Promotes Early Brain Injury After Subarachnoid Hemorrhage via Activating M1 Microglia. Transl. Stroke Res. 15, 1–15 (2024). https://doi.org/10.1007/s12975-022-01105-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01105-5