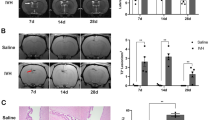
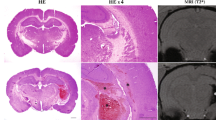
Abstract
Posthemorrhagic hydrocephalus occurs in up to 30% of infants with high-grade intraventricular hemorrhage and is associated with the worst neurocognitive outcomes in preterm infants. The mechanisms of posthemorrhagic hydrocephalus after intraventricular hemorrhage are unknown; however, CSF levels of iron metabolic pathway proteins including hemoglobin have been implicated in its pathogenesis. Here, we develop an animal model of intraventricular hemorrhage using intraventricular injection of hemoglobin at post-natal day 4 that results in acute and chronic hydrocephalus, pathologic choroid plexus iron accumulation, and subsequent choroid plexus injury at post-natal days 5, 7, and 15. This model also results in increased expression of aquaporin-1, Na+/K+/Cl- cotransporter 1, and Na+/K+/ATPase on the apical surface of the choroid plexus 24 h post-intraventricular hemorrhage. We use this model to evaluate a clinically relevant treatment strategy for the prevention of neurological sequelae after intraventricular hemorrhage using intraventricular administration of the iron chelator deferoxamine at the time of hemorrhage. Deferoxamine treatment prevented posthemorrhagic hydrocephalus for up to 11 days after intraventricular hemorrhage and prevented the development of sensorimotor gating deficits. In addition, deferoxamine treatment facilitated acute iron clearance through the choroid plexus and subsequently reduced choroid plexus iron levels at 24 h with reversal of hemoglobin-induced aquaporin-1 upregulation on the apical surface of the choroid plexus. Intraventricular administration of deferoxamine at the time of intraventricular hemorrhage may be a clinically relevant treatment strategy for preventing posthemorrhagic hydrocephalus and likely acts through promoting iron clearance through the choroid plexus to prevent hemoglobin-induced injury.








Similar content being viewed by others
Data Availability
The data that support the findings of this study may be available at the discretion of the corresponding author, upon reasonable request.
Abbreviations
- GMH:
-
Germinal matrix hemorrhage
- IVH:
-
Intraventricular hemorrhage
- HB:
-
Hemoglobin
- DFX:
-
Deferoxamine
- PHH:
-
Posthemorrhagic hydrocephalus
- ChP:
-
Choroid plexus
- LV:
-
Lateral ventricle
- AuNP:
-
Gold nanoparticle
- PBS:
-
Phosphate buffered saline
- PFA:
-
Paraformaldehyde
- H&E:
-
Hematoxylin and eosin
- AQP1:
-
Aquaporin-1
- NKAT:
-
Sodium potassium ATPase
- NKCC1:
-
Sodium potassium chloride cotransporter
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- TUNEL:
-
Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling
- EPM:
-
Elevated plus maze
- PPI:
-
Prepulse inhibition
- MWM:
-
Morris water maze
- SEM:
-
Standard error of the mean
- rm:
-
Repeated measures
- CSP:
-
Cavum septum pellucidum
References
Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8.
Strahle JM, Triplett RL, Alexopoulos D, Smyser TA, Rogers CE, Limbrick DD, et al. Impaired hippocampal development and outcomes in very preterm infants with perinatal brain injury. NeuroImage Clinical. 2019;22:101878.
Christian EA, Melamed EF, Peck E, Krieger MD, McComb JG. Surgical management of hydrocephalus secondary to intraventricular hemorrhage in the preterm infant. J Neurosurg Pediatr. 2016;17(3):278–84.
Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab. 2014;34(6):1070–5.
Garton T, Keep RF, Hua Y, Xi G. Brain iron overload following intracranial haemorrhage. Stroke Vasc Neurol. 2016;1(4):172–84.
Garton TP, He Y, Garton HJL, Keep RF, Xi G, Strahle JM. Hemoglobin-induced neuronal degeneration in the hippocampus after neonatal intraventricular hemorrhage. Brain Res. 2016;1635:86–94.
Mahaney KB, Buddhala C, Paturu M, Morales D, Limbrick DD, Strahle JM. Intraventricular hemorrhage clearance in human neonatal cerebrospinal fluid: associations with hydrocephalus. Stroke. 2020;51(6):1712–9.
Strahle JM, Garton T, Bazzi AA, Kilaru H, Garton HJL, Maher CO, et al. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurg. 2014;75(6):696–706.
Strahle JM, Mahaney KB, Morales DM, Buddhala C, Shannon CN, Wellons JC, et al. Longitudinal CSF iron pathway proteins in posthemorrhagic hydrocephalus: associations with ventricle size and neurodevelopmental outcomes. Annal Neurol. 2021;90:2.
Ballabh P, de Vries LS. White matter injury in infants with intraventricular haemorrhage: mechanisms and therapies. Nature Rev Neurol. 2021;17(4):199–214.
Merhar SL, Tabangin ME, Meinzen-Derr J, Schibler KR. Grade and laterality of intraventricular haemorrhage to predict 18–22 month neurodevelopmental outcomes in extremely low birthweight infants. Acta Paediat Intl J Paediatr. 2012;101(4):414–8.
Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, et al. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Edit. 2002;87(1):37F – 41.
Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–24.
Cherian SS, Love S, Silver IA, Porter HJ, Whitelaw AGL, Thoresen M. Posthemorrhagic ventricular dilation in the neonate: development and characterization of a rat model. J Neuropathol Exp Neurol. 2003;62(3):292–303.
Wan S, Hua Y, Keep RF, Hoff JT, Xi G. Deferoxamine reduces CSF free iron levels following intracerebral hemorrhage. Acta Neurochir Suppl. 2006;96:199–202.
Gozzelino R, Arosio P. Iron homeostasis in health and disease. Intl J Mol Sci. 2016;17(1):130.
Klebe D, Krafft PR, Hoffmann C, Lekic T, Flores JJ, Rolland W, et al. Acute and delayed Deferoxamine treatment attenuates long-term sequelae after germinal matrix hemorrhage in neonatal rats. Stroke. 2014;45(8):2475–9.
LeBlanc RH, Chen R, Selim MH, Hanafy KA. Heme oxygenase-1-mediated neuroprotection in subarachnoid hemorrhage via intracerebroventricular deferoxamine. J. Neuroinflammation. 2016;13(1):244.
Lekic T, Klebe D, Pichon P, Brankov K, Sultan S, McBride D, et al. Aligning animal models of clinical germinal matrix hemorrhage, from basic correlation to therapeutic approach. Curr. Drug Targets. 2016;18(12):1316–1328.
Mackenzie EL, Iwasaki K, Tsuji Y. Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid. Redox Signaling. 2008;10(6):997–1030.
Meng H, Li F, Hu R, Yuan Y, Gong G, Hu S, et al. Deferoxamine alleviates chronic hydrocephalus after intraventricular hemorrhage through iron chelation and Wnt1/Wnt3a inhibition. Brain Res. 2015;1602(44):52.
Mobarra N, Shanaki M, Ehteram H, Nasiri H, Sahmani M, Saeidi M, et al. A review on iron chelators in treatment of iron overload syndromes. Int J Hematol Oncol Stem Cell Res. 2016;10(4):239–247.
Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30(1):11–26.
Sun A, Wang J. Choroid plexus and drug removal mechanisms. AAPS Journal. 2021;23(3):61.
Zhang DL, Ghosh MC, Rouault TA. The physiological functions of iron regulatory proteins in iron homeostasis—an update. Front. Pharmacol. 2014;5:124.
Chen Q, Tang J, Tan L, Guo J, Tao Y, Li L, et al. Intracerebral hematoma contributes to hydrocephalus after intraventricular hemorrhage via aggravating iron accumulation. Stroke. 2015;46(10):2902–8.
Zhang J, Shi X, Chen Z, Geng J, Wang Y, Feng H, et al. Edaravone reduces iron-mediated hydrocephalus and behavioral disorder in rat by activating the Nrf2/HO-1 pathway. J. Stroke Cerebrovasc Dis. 2018;27(12):3511–3520.
Dang S, Rasmussen CA, LeVine SM. Immunocytochemical localization of desferrioxamine in the kidney, liver and brain of the developing and adult mouse: implications for drug processing and therapeutic mechanisms. Res Commun Mol Pathol Pharmacol. 1994 Oct;86(1):43-57.
Xu H, Fame RM, Sadegh C, Sutin J, Naranjo C, della Syau, et al. Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development. Nat Commun 2021;12(1):447
Okubo S, Strahle J, Keep RF, Hua Y, Xi G. Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke. 2013;44(2):547-550.
Klebe D, McBride D, Krafft PR, Flores JJ, Tang J, Zhang JH. Posthemorrhagic hydrocephalus development after germinal matrix hemorrhage: established mechanisms and proposed pathways. J Neurosci Res. 2020;98(1):105–120.
Li Q, Ding Y, Krafft P, Wan W, Yan F, Wu G, et al. Targeting germinal matrix hemorrhage-induced overexpression of sodium-coupled bicarbonate exchanger reduces Posthemorrhagic hydrocephalus formation in neonatal rats. J Am Heart Assoc. 2018;7(3):e007192.
Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106–107:1–16.
Ding Y, Zhang T, Wu G, McBride DW, Xu N, Klebe DW, et al. Astrogliosis inhibition attenuates hydrocephalus by increasing cerebrospinal fluid reabsorption through the glymphatic system after germinal matrix hemorrhage. Exp Neurol. 2019;320:113003.
Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G. Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb. Blood Flow Metab. 2014;34(3):489–494.
Longatti PL, Basaldella L, Orvieto E, Fiorindi A, Carteri A. Choroid plexus and aquaporin-1: a novel explanation of cerebrospinal fluid production. Pediatr Neurosurg. 2004;40(6):277–83.
Strahle J, Garton HJL, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl. Stroke Res. 2012;(Suppl 1):25–38.
Rouault TA, Zhang DL, Jeong SY. Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metab Brain Dis. 2009;24(4):673–684.
Alvira-Botero X, M. Carro E. Clearance of amyloid-β peptide across the choroid plexus in Alzheimers disease. Curr. Aging Sci. 2010;3(3):219-29.
Ogawa M, Suzuki H, Sawada Y, Hanano M, Sugiyama Y. Kinetics of active efflux via choroid plexus of β-lactam antibiotics from the CSF into the circulation. Am J Physiol Regul Integr Comp Physiol. 1994;266(2 Pt 2):R392–9.
Schweizer C, Fraering PC, Kühn LC. Ferritin H gene deletion in the choroid plexus and forebrain results in hydrocephalus. Neurochem Int. 2014;71:17–21.
Eide PK, Valnes LM, Pripp AH, Mardal KA, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from choroid plexus in idiopathic normal pressure hydrocephalus. J. Cereb. Blood Flow Metab. 2020;40(9):1849–1858.
Tadayon E, Pascual-Leone A, Press D, Santarnecchi E. Choroid plexus volume is associated with levels of CSF proteins: relevance for Alzheimer’s and Parkinson’s disease. Neurobiol Aging. 2020;89:108–117.
Ghaffari H, Grant SC, Petzold LR, Harrington MG. Regulation of CSF and brain tissue sodium levels by the blood-CSF and blood-brain barriers during migraine. Front Comput Neurosci. 2020;14:4.
Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017 Aug;23(8):997–1003.
Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Research. 2008;5:10.
Castañeyra-Ruiz L, Hernández-Abad LG, Carmona-Calero EM, Castañeyra-Perdomo A, González-Marrero I. AQP1 overexpression in the CSF of obstructive hydrocephalus and inversion of its polarity in the choroid plexus of a Chiari malformation type II case. J Neuropathol Exp Neurol. 2019;78(7):641–647.
Casey JL, Koeller DM, Ramin VC, Klausner RD, Harford JB. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3’ untranslated region of the mRNA. EMBO Journal. 1989;8(12):3693–3699.
Hentze MW, Caughman SW, Rouault TA, Barriocanal JG, Dancis A, Harford JB, et al. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987;238(4833):1570–3.
Fisahn A, Lavebratt C, Canlon B. Acoustic startle hypersensitivity in Mceph mice and its effect on hippocampal excitability. Eur J Neurosci. 2011;34(7):1121–30.
Rezayat M, Roohbakhsh A, Zarrindast MR, Massoudi R, Djahanguiri B. Cholecystokinin and GABA interaction in the dorsal hippocampus of rats in the elevated plus-maze test of anxiety. Physiol Behav. 2005;84(5):775–82.
Swerdlow NR, Light GA, Breier MR, Shoemaker JM, saint Marie RL, Neary AC, et al. Sensory and sensorimotor gating deficits after neonatal ventral hippocampal lesions in rats. Dev Neurosci. 2012;34(2-3):240–9.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82.
Kaur C, Ling EA. Transitory cystic cavities in the developing mammalian brain – normal or anomalous? Journal of Anatomy. 2017;230(2):197–202.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates seventh edition. Elsevier Academic Press. 2014;170.
Maloney SE, Yuede CM, Creeley CE, Williams SL, Huffman JN, Taylor GT, et al. Repeated neonatal isoflurane exposures in the mouse induce apoptotic degenerative changes in the brain and relatively mild long-term behavioral deficits. Sci Rep. 2019;9(1):2779.
Palanisamy A, Giri T, Jiang J, Bice A, Quirk JD, Conyers SB, et al. In utero exposure to transient ischemia-hypoxemia promotes long-term neurodevelopmental abnormalities in male rat offspring. JCI Insight. 2020;5(10):e133172.
Acknowledgements
The studies presented in this work were carried out, in part, using the Small Animal MR Facility of the Mallinckrodt Institute of Radiology, Hope Center Alafi Neuroimaging Lab, and Washington University Center for Cellular Imaging (WUCCI), supported by Washington University in St. Louis School of Medicine.
Funding
This study is funded by NIH R01 NS110793 (JMS), K12 Neurosurgeon Research Career Development Program (JMS), Hydrocephalus Association (JMS), WUCCI, McDonnell Centers for Systems Neuroscience and Cellular Neurobiology (JMS, DFW), Children’s Discovery Institute of Washington University, and St. Louis Children’s Hospital (JMS). The MRI studies presented in this work were carried out in the Small Animal MR Facility of the Mallinckrodt Institute of Radiology at Washington University (18–019, JMS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramagiri, S., Pan, S., DeFreitas, D. et al. Deferoxamine Prevents Neonatal Posthemorrhagic Hydrocephalus Through Choroid Plexus-Mediated Iron Clearance. Transl. Stroke Res. 14, 704–722 (2023). https://doi.org/10.1007/s12975-022-01092-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01092-7