Abstract
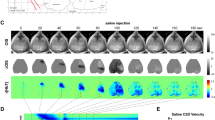
Delayed cerebral ischemia (DCI) is the most severe complication after subarachnoid hemorrhage (SAH), and cortical spreading depolarization (CSD) is believed to play a vital role in it. However, the dynamic changes in cerebral blood flow (CBF) in response to CSD in typical SAH models have not been well investigated. Here, SAH was established in mice with endovascular perforation. Subsequently, the spontaneous CBF dropped instantly and then returned to baseline rapidly. After KCl application to the cortex, subsequent hypoperfusion waves occurred across the groups, while a lower average perfusion level was found in the SAH groups (days 1–7). Moreover, in the SAH groups, the number of CSD decreased within day 7, and the duration and spreading velocity of the CSD increased within day 3 and day 14, respectively. Next, we continuously monitored the local field potential (LFP) in the prefrontal cortex. The results showed that the decrease in the percentage of gamma oscillations lasted throughout the whole process in the SAH group. In the chronic phase after SAH, we found that the mice still had cognitive deficits but experienced no obvious tissue damage. In summary, SAH negatively affects the CBF responses to CSD and the spontaneous LFP activity and causes long-term cognitive deficits in mice. Based on these findings, in the specific phase after SAH, DCI is induced or exacerbated more easily by potential causers of CSD in clinical practice (edema, erythrocytolysis, inflammation), which may lead to neurological deterioration.







Similar content being viewed by others
References
Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet (London, England). 2017;389(10069):655–66. https://doi.org/10.1016/s0140-6736(16)30668-7.
Neifert SN, Chapman EK, Martini ML, Shuman WH, Schupper AJ, Oermann EK, et al. Aneurysmal subarachnoid hemorrhage: the last decade. Transl Stroke Res. 2021;12(3):428–46. https://doi.org/10.1007/s12975-020-00867-0.
Megjhani M, Terilli K, Weiss M, Savarraj J, Chen LH, Alkhachroum A, et al. Dynamic detection of delayed cerebral ischemia: a study in 3 centers. Stroke. 2021;52(4):1370–9. https://doi.org/10.1161/strokeaha.120.032546.
Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care. 2016;20(1):277. https://doi.org/10.1186/s13054-016-1447-6.
Brathwaite S, Macdonald RL. Current management of delayed cerebral ischemia: update from results of recent clinical trials. Transl Stroke Res. 2014;5(2):207–26. https://doi.org/10.1007/s12975-013-0316-8.
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. https://doi.org/10.1038/nrneurol.2013.246.
Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439–47. https://doi.org/10.1038/nm.2333.
Ayata C, Lauritzen M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev. 2015;95(3):953–93. https://doi.org/10.1152/physrev.00027.2014.
Dreier JP, Lemale CL, Kola V, Friedman A, Schoknecht K. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology. 2018;134(Pt B):189–207. https://doi.org/10.1016/j.neuropharm.2017.09.027.
Kirov SA, Fomitcheva IV, Sword J. Rapid neuronal ultrastructure disruption and recovery during spreading depolarization-induced cytotoxic edema. Cerebral cortex (New York, NY: 1991). 2020;30(10):5517–31. https://doi.org/10.1093/cercor/bhaa134.
Lemale CL, Lückl J, Horst V, Reiffurth C, Major S, Hecht N, et al. Migraine aura, transient ischemic attacks, stroke, and dying of the brain share the same key pathophysiological process in neurons driven by Gibbs-Donnan forces, namely spreading depolarization. Front Cell Neurosci. 2022;16: 837650. https://doi.org/10.3389/fncel.2022.837650.
Dreier JP, Körner K, Ebert N, Görner A, Rubin I, Back T, et al. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cerebral Blood Flow Metab : Off J Int Soc Cerebral Blood Flow Metab. 1998;18(9):978–90. https://doi.org/10.1097/00004647-199809000-00007.
Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain : a journal of neurology. 2009;132(Pt 7):1866–81. https://doi.org/10.1093/brain/awp102.
Dreier JP, Ebert N, Priller J, Megow D, Lindauer U, Klee R, et al. Products of hemolysis in the subarachnoid space inducing spreading ischemia in the cortex and focal necrosis in rats: a model for delayed ischemic neurological deficits after subarachnoid hemorrhage? J Neurosurg. 2000;93(4):658–66. https://doi.org/10.3171/jns.2000.93.4.0658.
Dreier JP, Petzold G, Tille K, Lindauer U, Arnold G, Heinemann U, et al. Ischaemia triggered by spreading neuronal activation is inhibited by vasodilators in rats. J Physiol. 2001;531(Pt 2):515–26. https://doi.org/10.1111/j.1469-7793.2001.0515i.x.
Dreier JP, Windmüller O, Petzold G, Lindauer U, Einhäupl KM, Dirnagl U. Ischemia triggered by red blood cell products in the subarachnoid space is inhibited by nimodipine administration or moderate volume expansion/hemodilution in rats. Neurosurgery. 2002;51(6):1457–65; discussion 65–7.
Hartings JA, Strong AJ, Fabricius M, Manning A, Bhatia R, Dreier JP, et al. Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma. 2009;26(11):1857–66. https://doi.org/10.1089/neu.2009.0961.
Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81(3):1065–96. https://doi.org/10.1152/physrev.2001.81.3.1065.
Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10(6):754–62. https://doi.org/10.1038/nn1902.
Lückl J, Lemale CL, Kola V, Horst V, Khojasteh U, Oliveira-Ferreira AI, et al. The negative ultraslow potential, electrophysiological correlate of infarction in the human cortex. Brain : a journal of neurology. 2018;141(6):1734–52. https://doi.org/10.1093/brain/awy102.
Hartings JA, York J, Carroll CP, Hinzman JM, Mahoney E, Krueger B, et al. Subarachnoid blood acutely induces spreading depolarizations and early cortical infarction. Brain. 2017;140(10):2673–90. https://doi.org/10.1093/brain/awx214.
Hamming AM, Wermer MJH, Umesh Rudrapatna S, Lanier C, van Os HJA, van den Bergh WM, et al. Spreading depolarizations increase delayed brain injury in a rat model of subarachnoid hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016;36(7):1224–31. https://doi.org/10.1177/0271678x15619189.
Tang Y, She D, Li P, Pan L, Lu J, Liu P. Cortical spreading depression aggravates early brain injury in a mouse model of subarachnoid hemorrhage. J Biophotonics. 2021;14(4): e202000379. https://doi.org/10.1002/jbio.202000379.
Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129(Pt 12):3224–37. https://doi.org/10.1093/brain/awl297.
Kawano A, Sugimoto K, Nomura S, Inoue T, Kawano R, Oka F, et al. Association between spreading depolarization and delayed cerebral ischemia after subarachnoid hemorrhage: post hoc analysis of a randomized trial of the effect of cilostazol on delayed cerebral ischemia. Neurocrit Care. 2021;35(Suppl 2):91–9. https://doi.org/10.1007/s12028-021-01330-0.
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410. https://doi.org/10.1371/journal.pbio.3000410.
Pang J, Peng J, Matei N, Yang P, Kuai L, Wu Y, et al. Apolipoprotein E exerts a whole-brain protective property by promoting M1? Microglia quiescence after experimental subarachnoid hemorrhage in mice. Transl Stroke Res. 2018;9(6):654–68. https://doi.org/10.1007/s12975-018-0665-4.
Liu H, He J, Zhang Z, Liu L, Huo G, Sun X, et al. Evolution of cerebral perfusion in the peri-contusional cortex in mice revealed by in vivo laser speckle imaging after traumatic brain injury. Brain Res. 2018;1700:118–25. https://doi.org/10.1016/j.brainres.2018.07.006.
Obrenovitch TP, Chen S, Farkas E. Simultaneous, live imaging of cortical spreading depression and associated cerebral blood flow changes, by combining voltage-sensitive dye and laser speckle contrast methods. Neuroimage. 2009;45(1):68–74. https://doi.org/10.1016/j.neuroimage.2008.11.025.
Lin L, Chen G, Xie K, Zaia KA, Zhang S, Tsien JZ. Large-scale neural ensemble recording in the brains of freely behaving mice. J Neurosci Methods. 2006;155(1):28–38. https://doi.org/10.1016/j.jneumeth.2005.12.032.
Liu L, Deng H, Tang X, Lu Y, Zhou J, Wang X, et al. Specific electromagnetic radiation in the wireless signal range increases wakefulness in mice. Proc Natl Acad Sci U S A. 2021;118(31). https://doi.org/10.1073/pnas.2105838118.
Zhou C, Chen H, Zheng JF, Guo ZD, Huang ZJ, Wu Y, et al. Pentraxin 3 contributes to neurogenesis after traumatic brain injury in mice. Neural Regen Res. 2020;15(12):2318–26. https://doi.org/10.4103/1673-5374.285001.
Chen S, Peng J, Sherchan P, Ma Y, Xiang S, Yan F, et al. TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice. J Neuroinflammation. 2020;17(1):168. https://doi.org/10.1186/s12974-020-01853-x.
Carlén M. What constitutes the prefrontal cortex? Science. 2017;358(6362):478–82. https://doi.org/10.1126/science.aan8868.
Lai JH, Qin T, Sakadžić S, Ayata C, Chung DY. Cortical spreading depolarizations in a mouse model of subarachnoid hemorrhage. Neurocrit Care. 2022. https://doi.org/10.1007/s12028-021-01397-9.
Zheng Z, Schoell M, Sanchez-Porras R, Diehl C, Unterberg A, Sakowitz OW. Spreading depolarization during the acute stage of experimental subarachnoid hemorrhage in mice. Acta Neurochir Suppl. 2020;127:97–103. https://doi.org/10.1007/978-3-030-04615-6_16.
Oka F, Hoffmann U, Lee JH, Shin HK, Chung DY, Yuzawa I, et al. Requisite ischemia for spreading depolarization occurrence after subarachnoid hemorrhage in rodents. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2017;37(5):1829–40. https://doi.org/10.1177/0271678x16659303.
Conzen C, Becker K, Albanna W, Weiss M, Bach A, Lushina N, et al. The acute phase of experimental subarachnoid hemorrhage: intracranial pressure dynamics and their effect on cerebral blood flow and autoregulation. Transl Stroke Res. 2019;10(5):566–82. https://doi.org/10.1007/s12975-018-0674-3.
Westermaier T, Jauss A, Eriskat J, Kunze E, Roosen K. Time-course of cerebral perfusion and tissue oxygenation in the first 6 h after experimental subarachnoid hemorrhage in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29(4):771–9. https://doi.org/10.1038/jcbfm.2008.169.
Megyesi JF, Vollrath B, Cook DA, Findlay JM. In vivo animal models of cerebral vasospasm: a review. Neurosurgery. 2000;46(2):448–60; discussion 60–1.
van den Maagdenberg AM, Pizzorusso T, Kaja S, Terpolilli N, Shapovalova M, Hoebeek FE, et al. High cortical spreading depression susceptibility and migraine-associated symptoms in Ca(v)2.1 S218L mice. Ann Neurol. 2010;67(1):85–98. https://doi.org/10.1002/ana.21815.
Merkler D, Klinker F, Jürgens T, Glaser R, Paulus W, Brinkmann BG, et al. Propagation of spreading depression inversely correlates with cortical myelin content. Ann Neurol. 2009;66(3):355–65. https://doi.org/10.1002/ana.21746.
Milakara D, Grozea C, Dahlem M, Major S, Winkler MKL, Lückl J, et al. Simulation of spreading depolarization trajectories in cerebral cortex: correlation of velocity and susceptibility in patients with aneurysmal subarachnoid hemorrhage. NeuroImage Clinical. 2017;16:524–38. https://doi.org/10.1016/j.nicl.2017.09.005.
Ohta OOK SM, Yamamoto M, Shimizu K, Toda N. Cerebral vasospasm following ruptured intracranial aneurysms, especially some contributions of potassium ion released from subarachnoid hematoma to delayed cerebral vasospasm. In: JA B, editor. In Vascular Neuroeffector Mechanisms: 4th International Symposium. New York: Raven1983. p. 353 – 8.
Antunes AP, Schiefecker AJ, Beer R, Pfausler B, Sohm F, Fischer M, et al. Higher brain extracellular potassium is associated with brain metabolic distress and poor outcome after aneurysmal subarachnoid hemorrhage. Crit Care. 2014;18(3):R119. https://doi.org/10.1186/cc13916.
Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22(8):971–82. https://doi.org/10.1161/01.str.22.8.971.
Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep. 2017;19(12):50. https://doi.org/10.1007/s11883-017-0690-x.
Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents–EEG, ECoG. LFP and spikes Nat Rev Neurosci. 2012;13(6):407–20. https://doi.org/10.1038/nrn3241.
Yamamoto J, Suh J, Takeuchi D, Tonegawa S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell. 2014;157(4):845–57. https://doi.org/10.1016/j.cell.2014.04.009.
Gao N, Wang H, Xu X, Yang Z, Zhang T. Angiotensin II induces cognitive decline and anxiety-like behavior via disturbing pattern of theta-gamma oscillations. Brain Res Bull. 2021;174:84–91. https://doi.org/10.1016/j.brainresbull.2021.06.002.
Sauer JF, Strüber M, Bartos M. Impaired fast-spiking interneuron function in a genetic mouse model of depression. Elife. 2015;4. https://doi.org/10.7554/eLife.04979.
Cao W, Lin S, Xia QQ, Du YL, Yang Q, Zhang MY, et al. Gamma oscillation dysfunction in mPFC leads to social deficits in neuroligin 3 R451C knockin mice. Neuron. 2018;97(6):1253-60.e7. https://doi.org/10.1016/j.neuron.2018.02.001.
Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–36. https://doi.org/10.1161/strokeaha.110.581975.
Regnier-Golanov AS, Gulinello M, Hernandez MS, Golanov EV, Britz GW. Subarachnoid hemorrhage induces sub-acute and early chronic impairment in learning and memory in mice. Transl Stroke Res. 2022. https://doi.org/10.1007/s12975-022-00987-9.
Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron. 2015;86(4):902–22. https://doi.org/10.1016/j.neuron.2015.04.004.
Acknowledgements
We would like to thank the service provided by the Chongqing Key Laboratory of Neurology (Chongqing, China).
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by the National Natural Science Foundation of China, No. 82172193 to Dr Cheng; the National Natural Science Foundation of China, No. 82071332 to Dr Guo; and the Natural Science Foundation of Chongqing, China, No. cstc2019ycyj-msxmX0830 to Dr Xia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
All procedures on animals were approved by the Ethics Committee of Chongqing Medical University and carried out in accordance with ARRIVE guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (AVI 35906 KB)
Supplementary file3 (AVI 22864 KB)
Supplementary file4 (AVI 24602 KB)
Rights and permissions
About this article
Cite this article
Yan, J., Li, W., Zhou, C. et al. Dynamic Measurements of Cerebral Blood Flow Responses to Cortical Spreading Depolarization in the Murine Endovascular Perforation Subarachnoid Hemorrhage Model. Transl. Stroke Res. 14, 530–544 (2023). https://doi.org/10.1007/s12975-022-01052-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01052-1