Abstract
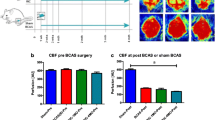
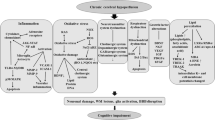
Vascular contributions to cognitive impairment and dementia (VCID) secondary to chronic mild-moderate cerebral ischemia underlie a significant percentage of cases of dementia. We previously reported that either genetic deficiency of the complement C3a receptor (C3aR) or its pharmacological inhibition protects against cerebral ischemia in rodents, while others have implicated C3aR in the pathogenesis seen in rodent transgenic models of Alzheimer’s disease. In the present study, we evaluated the role of complement C3a-C3aR signaling in the onset and progression of VCID. We utilized the bilateral common carotid artery stenosis (BCAS) model to induce VCID in male C57BL/6 wild-type and C3aR-knockout (C3aR−/−) mice. Cerebral blood flow (CBF) changes, hippocampal atrophy (HA), white matter degeneration (WMD), and ventricular size were assessed at 4 months post-BCAS using laser speckle contrast analysis (LSCI) and magnetic resonance imaging (MRI). Cognitive function was evaluated using the Morris water maze (MWM), and novel object recognition (NOR), immunostaining, and western blot were performed to assess the effect of genetic C3aR deletion on post-VCID outcomes. BCAS resulted in decreased CBF and increased HA, WMD, and neurovascular inflammation in WT (C57BL/6) compared to C3aR−/− (C3aR-KO) mice. Moreover, C3aR−/− mice exhibited improved cognitive function on NOR and MWM relative to WT controls. We conclude that over-activation of the C3a/C3aR axis exacerbates neurovascular inflammation leading to poor VCID outcomes which are mitigated by C3aR deletion. Future studies are warranted to dissect the role of cell-specific C3aR in VCID.









Similar content being viewed by others
References
Corriveau RA, Bosetti F, Emr M, Gladman JT, Koenig JI, Moy CS, et al. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. 2016;36(2):281–8.
Khan MB, Hafez S, Hoda MN, Baban B, Wagner J, Awad ME, et al. Chronic remote ischemic conditioning is cerebroprotective and induces vascular remodeling in a VCID model. Transl Stroke Res. 2018;9(1):51–63.
Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, et al. Vascular cognitive impairment and dementia: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73(25):3326–44.
Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–713.
O’Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–706.
Matsui Y, Tanizaki Y, Arima H, Yonemoto K, Doi Y, Ninomiya T, et al. Incidence and survival of dementia in a general population of Japanese elderly: the Hisayama study. J Neurol Neurosurg Psychiatry. 2009;80(4):366–70.
Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015;11(6):710–7.
Nishio K, Ihara M, Yamasaki N, Kalaria RN, Maki T, Fujita Y, et al. A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke. 2010;41(6):1278–84.
Shibata M, Yamasaki N, Miyakawa T, Kalaria RN, Fujita Y, Ohtani R, et al. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke. 2007;38(10):2826–32.
Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35(11):2598–603.
Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–66.
de la Torre JC, Aliev G. Inhibition of vascular nitric oxide after rat chronic brain hypoperfusion: spatial memory and immunocytochemical changes. J Cereb Blood Flow Metab. 2005;25(6):663–72.
Liu Q, He S, Groysman L, Shaked D, Russin J, Scotton TC, et al. White matter injury due to experimental chronic cerebral hypoperfusion is associated with C5 deposition. PLoS One. 2013;8(12):e84802.
Kitaguchi H, Tomimoto H, Ihara M, Shibata M, Uemura K, Kalaria RN, et al. Chronic cerebral hypoperfusion accelerates amyloid beta deposition in APPSwInd transgenic mice. Brain Res. 2009;1294:202–10.
O’Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59(3):321–6.
Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5(5):347–60.
Promjunyakul N, Lahna D, Kaye JA, Dodge HH, Erten-Lyons D, Rooney WD, et al. Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. Neuroimage Clin. 2015;8:224–9.
Promjunyakul NO, Lahna DL, Kaye JA, Dodge HH, Erten-Lyons D, Rooney WD, et al. Comparison of cerebral blood flow and structural penumbras in relation to white matter hyperintensities: a multi-modal magnetic resonance imaging study. J Cereb Blood Flow Metab. 2016;36(9):1528–36.
de Vries HE, Kooij G, Frenkel D, Georgopoulos S, Monsonego A, Janigro D. Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia. 2012;53(Suppl 6):45–52.
Wang F, Cao Y, Ma L, Pei H, Rausch WD, Li H. Dysfunction of cerebrovascular endothelial cells: prelude to vascular dementia. Front Aging Neurosci. 2018;10:376.
Manukjan N, Ahmed Z, Fulton D, Blankesteijn WM, Foulquier S. A systematic review of WNT signaling in endothelial cell oligodendrocyte interactions: potential relevance to cerebral small vessel disease. Cells. 2020;9(6):1545.
Alafuzoff I, Adolfsson R, Grundke-Iqbal I, Winblad B. Blood-brain barrier in Alzheimer dementia and in non-demented elderly. An immunocytochemical study. Acta Neuropathol. 1987;73(2):160–6.
Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging. 2009;30(3):337–52.
Schreiber S, Bueche CZ, Garz C, Braun H. Blood brain barrier breakdown as the starting point of cerebral small vessel disease? - New insights from a rat model. Exp Transl Stroke Med. 2013;5(1):4.
Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, et al. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer’s disease and vascular dementia. Neurology. 1998;50(4):966–71.
Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201.
Sewell DL, Nacewicz B, Liu F, Macvilay S, Erdei A, Lambris JD, et al. Complement C3 and C5 play critical roles in traumatic brain cryoinjury: blocking effects on neutrophil extravasation by C5a receptor antagonist. J Neuroimmunol. 2004;155(1–2):55–63.
Mocco J, Mack WJ, Ducruet AF, Sosunov SA, Sughrue ME, Hassid BG, et al. Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99(2):209–17.
Engstrom G, Hedblad B, Janzon L, Lindgarde F. Complement C3 and C4 in plasma and incidence of myocardial infarction and stroke: a population-based cohort study. Eur J Cardiovasc Prev Rehabil. 2007;14(3):392–7.
Cojocaru IM, Cojocaru M, Tanasescu R, Burcin C, Atanasiu AN, Petrescu AM, et al. Changes in plasma levels of complement in patients with acute ischemic stroke. Rom J Intern Med. 2008;46(1):77–80.
Ducruet AF, Hassid BG, Mack WJ, Sosunov SA, Otten ML, Fusco DJ, et al. C3a receptor modulation of granulocyte infiltration after murine focal cerebral ischemia is reperfusion dependent. J Cereb Blood Flow Metab. 2008;28(5):1048–58.
Rynkowski MA, Kim GH, Garrett MC, Zacharia BE, Otten ML, Sosunov SA, et al. C3a receptor antagonist attenuates brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29(1):98–107.
Ducruet AF, Zacharia BE, Sosunov SA, Gigante PR, Yeh ML, Gorski JW, et al. Complement inhibition promotes endogenous neurogenesis and sustained anti-inflammatory neuroprotection following reperfused stroke. PLoS One. 2012;7(6):e38664.
Stokowska A, Olsson S, Holmegaard L, Jood K, Blomstrand C, Jern C, et al. Cardioembolic and small vessel disease stroke show differences in associations between systemic C3 levels and outcome. PLoS One. 2013;8(8):e72133.
Zhang LY, Pan J, Mamtilahun M, Zhu Y, Wang L, Venkatesh A, et al. Microglia exacerbate white matter injury via complement C3/C3aR pathway after hypoperfusion. Theranostics. 2020;10(1):74–90.
Zhang B, Yang N, Gao C. Is plasma C3 and C4 levels useful in young cerebral ischemic stroke patients? Associations with prognosis at 3 months. J Thromb Thrombolysis. 2015;39(2):209–14.
Propson NE, Roy ER, Litvinchuk A, Köhl J, Zheng H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J Clin Invest. 2021;131(1): e140966.
Litvinchuk A, Wan YW, Swartzlander DB, Chen F, Cole A, Propson NE, et al. Complement C3aR inactivation attenuates tau pathology and reverses an immune network deregulated in tauopathy models and Alzheimer’s disease. Neuron. 2018;100(6):1337-53.e5.
Ihara M, Tomimoto H. Lessons from a mouse model characterizing features of vascular cognitive impairment with white matter changes. J Aging Res. 2011;2011:978761.
Ahmad S, Kindelin A, Khan SA, Ahmed M, Hoda MN, Bhatia K, et al. C3a receptor inhibition protects brain endothelial cells against oxygen-glucose deprivation/reperfusion. Exp Neurobiol. 2019;28(2):216–28.
Ahmad S, Pandya C, Kindelin A, Bhatia K, Chaudhary R, Dwivedi AK, et al. C3a receptor antagonist therapy is protective with or without thrombolysis in murine thromboembolic stroke. Br J Pharmacol. 2020;177(11):2466–77.
Bhatia K, Ahmad S, Kindelin A, Ducruet AF. Complement C3a receptor-mediated vascular dysfunction: a complex interplay between aging and neurodegeneration. J Clin Invest. 2021;131(1): e144348.
Coulthard LG, Woodruff TM. Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J Immunol. 2015;194(8):3542–8.
Stokowska A, Atkins AL, Morán J, Pekny T, Bulmer L, Pascoe MC, et al. Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia. Brain. 2017;140(2):353–69.
De Vis JB, Hendrikse J, Bhogal A, Adams A, Kappelle LJ, Petersen ET. Age-related changes in brain hemodynamics; a calibrated MRI study. Hum Brain Mapp. 2015;36(10):3973–87.
del Ser T, Bermejo F, Portera A, Arredondo JM, Bouras C, Constantinidis J. Vascular dementia. A clinicopathological study. J Neurol Sci. 1990;96(1):1–17.
Scheel P, Puls I, Becker G, Schoning M. Volume reduction in cerebral blood flow in patients with vascular dementia. Lancet. 1999;354(9196):2137.
Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 2009;5(6):454–62.
Khan MB, Hoda MN, Vaibhav K, Giri S, Wang P, Waller JL, et al. Remote ischemic postconditioning: harnessing endogenous protection in a murine model of vascular cognitive impairment. Transl Stroke Res. 2015;6(1):69–77.
Hess DC, Khan MB, Hoda N, Morgan JC. Remote ischemic conditioning: a treatment for vascular cognitive impairment. Brain Circ. 2015;1(2):133–9.
Dalby RB, Eskildsen SF, Videbech P, Frandsen J, Mouridsen K, Sorensen L, et al. Oxygenation differs among white matter hyperintensities, intersected fiber tracts and unaffected white matter. Brain Commun. 2019;1(1):fcz033.
Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63(5):693–9.
Apostolova LG, Mosconi L, Thompson PM, Green AE, Hwang KS, Ramirez A, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging. 2010;31(7):1077–88.
Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, et al. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord. 2007;21(1):14–24.
Yousef H, Czupalla CJ, Lee D, Chen MB, Burke AN, Zera KA, et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat Med. 2019;25(6):988–1000.
Dulken BW, Buckley MT, Navarro Negredo P, Saligrama N, Cayrol R, Leeman DS, et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature. 2019;571(7764):205–10.
Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 2020;577(7790):399–404.
Lee JD, Taylor SM, Woodruff TM. Is the C3a receptor antagonist SB290157 a useful pharmacological tool? Br J Pharmacol. 2020;177(24):5677–8.
Acknowledgements
This work was supported by the Barrow Neurological Foundation and the Arizona Alzheimer’s Research Consortium to AFD. The Arizona Alzheimer’s Consortium (AAC) is greatly acknowledged to support this work. The authors would like to thank the staff of the Neuroscience Publications at Barrow Neurological Institute for providing the schematic figure.
Author information
Authors and Affiliations
Contributions
KB, AK, MN, JY, KM, MBK, KM and SA performed experiments, analyzed data, and drafted the manuscript; AF and GHT performed MRI imaging and analyzed data; SA, ASA, MCP, EJF, and MFW reviewed and edited the manuscript; AFD designed and oversaw the project, assisted in analyzing and interpreting the data, and edited the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All applicable institutional and national guidelines for the care and use of animals were followed.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12975_2022_993_MOESM1_ESM.pptx
Supplementary file1 (PPTX 3700 KB) Supplemental Figure 1. Representative images showing baseline cortical CBF before surgery. We monitored CBF in each group including Sham (WT), Sham (KO), BCAS (WT) and BCAS (KO) mice. There was no statistical significance noted between group comparisons. Supplemental Figure 2. Representative Luxol fast blue (LFB) staining for white matter degeneration. The intensity of LFB staining was significantly decreased in BCAS (WT) compared with Sham (WT). However, BACS (KO) group showed significant improvement vs BCAS (WT). A, Representative images (20x) were taken from cross sections of corpus callosum of each group of mice with LFB staining and its higher magnification (40x, scale bar=20μm) at 4 months after BCAS surgery. B, Histogram representing grading score of white matter degeneration in BCAS-WT and improvement in BCAS-KO mice. Data are expressed as Mean ± SD. Data was analyzed using one-Way ANOVA with Tukey’s post-hoc, n=5 (*** p < 0.001 vs Sham, *p= 0.05 vs BCAS-KO). Supplemental Figure 3. Immunoblotting gel images. A-F, Uncropped western blot images (MBP, VCAM1, Iba1, ZO1, pSTAT3 and Occludin) are presented with maker band denoted. Supplemental Figure 4. A, mouse brain (hippocampal region) was stained with only secondary antibody conjugated with FITC-488 for negative control immunofluorescence. B, mouse brain (hippocampal region) was stained with FITC-488 and primary C3aR antibody.
Rights and permissions
About this article
Cite this article
Bhatia, K., Kindelin, A., Nadeem, M. et al. Complement C3a Receptor (C3aR) Mediates Vascular Dysfunction, Hippocampal Pathology, and Cognitive Impairment in a Mouse Model of VCID. Transl. Stroke Res. 13, 816–829 (2022). https://doi.org/10.1007/s12975-022-00993-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-00993-x