Abstract
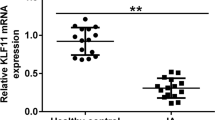
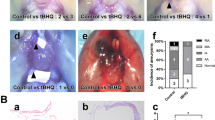
The objective of this study is to explore the role of the SDF-1α/CXCR4 pathway in the development of intracranial aneurysm (IA) induced by hemodynamic forces. We collected 12 IA and six superficial temporal artery samples for high-throughput sequencing, hematoxylin and eosin staining, and immunohistochemistry to examine vascular remodeling and determine the expression of the components of the SDF-1α/CXCR4 pathway, structural proteins (α-SMA and calponin) of vascular smooth muscle cells (VSMCs), and inflammatory factors (MMP-2 and TNF-α). Computational fluid dynamics (CFD) was used for hemodynamic analysis. Mouse IA model and dynamic co-culture model were established to explore the mechanism through which the SDF-1α/CXCR4 pathway regulates the phenotypic transformation of VSMCs in vivo and in vitro. We detected a significant elevation of SDF-1α and CXCR4 in IA, which was accompanied by vascular remodeling in the aneurysm wall (i.e., the upregulation of inflammatory factors, MMP-2 and TNF-α, and the downregulation of contractile markers, α-SMA and calponin). In addition, hemodynamic analysis revealed that compared with unruptured aneurysms, ruptured aneurysms were associated with lower wall shear stress and higher MMP-2 expression. In vivo and in vitro experiments showed that abnormal hemodynamics could activate the SDF-1α/CXCR4, P38, and JNK signaling pathways to induce the phenotypic transformation of VSMCs, leading to IA formation. Hemodynamics can induce the phenotypic transformation of VSMCs and cause IA by activating the SDF-1α/CXCR4 signaling pathway.





Similar content being viewed by others
Availability of Data and Material
All the data obtained are transparent and credible and all the materials used are authentic.
Code Availability
All the software packages were obtained through the formal way and complied with field standards.
References
Li MH, Chen SW, Li YD, Chen YC, Cheng YS, Hu DJ, et al. Prevalence of unruptured cerebral aneurysms in chinese adults aged 35 to 75 years: a cross-sectional study. Ann Intern Med. 2013;159:514–21.
Li J, Shen B, Ma C, Liu L, Ren L, Fang Y, et al. 3d contrast enhancement-mr angiography for imaging of unruptured cerebral aneurysms: a hospital-based prevalence study. PloS One. 2014;9:e114157.
Kang XK, Guo SF, Lei Y, Wei W, Liu HX, Huang LL, et al. Endovascular coiling versus surgical clipping for the treatment of unruptured cerebral aneurysms: direct comparison of procedure-related complications. Medicine. 2020;99:e19654.
Xu Z, Rui YN, Hagan JP, Kim DH. Intracranial aneurysms: pathology, genetics, and molecular mechanisms. NeuroMol Med. 2019;21:325–43.
Teixido J, Martinez-Moreno M, Diaz-Martinez M, Sevilla-Movilla S. The good and bad faces of the cxcr4 chemokine receptor. Int J Biochem Cell Biol. 2018;95:121–31.
Kodali R, Hajjou M, Berman AB, Bansal MB, Zhang S, Pan JJ, et al. Chemokines induce matrix metalloproteinase-2 through activation of epidermal growth factor receptor in arterial smooth muscle cells. Cardiovasc Res. 2006;69:706–15.
Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension (Dallas, Tex. : 1979). 2009;54:1337–44.
Chiu JJ, Chen LJ, Lee PL, Lee CI, Lo LW, Usami S, et al. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood. 2003;101:2667–74.
Salmon M, Spinosa M, Zehner ZE, Upchurch GR, Ailawadi G. Klf4, klf2, and zfp148 activate autophagy-related genes in smooth muscle cells during aortic aneurysm formation. Physiol Rep. 2019;7:e14058.
Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. 2016;12:699–713.
Krings T, Mandell DM, Kiehl TR, Geibprasert S, Tymianski M, Alvarez H, et al. Intracranial aneurysms: from vessel wall pathology to therapeutic approach. Nat Rev Neurol. 2011;7:547–59.
Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32:1659–76.
Wang G, Jacquet L, Karamariti E, Xu Q. Origin and differentiation of vascular smooth muscle cells. J Physiol. 2015;593:3013–30.
Kilic T, Sohrabifar M, Kurtkaya O, Yildirim O, Elmaci I, Gunel M, et al. Expression of structural proteins and angiogenic factors in normal arterial and unruptured and ruptured aneurysm walls. Neurosurgery. 2005;57:997–1007 (discussion 1997-1007).
Fan W, Liu Y, Li C, Qu X, Zheng G, Zhang Q, et al. microRNA-331–3p maintains the contractile type of vascular smooth muscle cells by regulating TNF-α and CD14 in intracranial aneurysm. Neuropharmacology. 2020;164:107858.
Mandelbaum M, Kolega J, Dolan JM, Siddiqui AH, Meng H. A critical role for proinflammatory behavior of smooth muscle cells in hemodynamic initiation of intracranial aneurysm. PloS One. 2013;8:e74357.
Starke RM, Chalouhi N, Ding D, Raper DM, Mckisic MS, Owens GK, Hasan DM, Medel R, Dumont AS. Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Transl Stroke Res. 2014;5(3):338–46.
Metaxa E, Tremmel M, Natarajan SK, Xiang J, Paluch RA, Mandelbaum M, et al. Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model. Stroke. 2010;41:1774–82.
Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke. 2007;38:1924–31.
Cebral JR, Meng H. Counterpoint: Realizing the clinical utility of computational fluid dynamics–closing the gap. AJNR Am J Neuroradiol. 2012;33:396–8.
Meng H, Tutino VM, Xiang J, Siddiqui A. High wss or low wss? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014;35:1254–62.
Li Y, Huang J, He X, Tang G, Tang YH, Liu Y, et al. Postacute stromal cell-derived factor-1alpha expression promotes neurovascular recovery in ischemic mice. Stroke. 2014;45:1822–9.
Hoh BL, Hosaka K, Downes DP, Nowicki KW, Wilmer EN, Velat GJ, et al. Stromal cell-derived factor-1 promoted angiogenesis and inflammatory cell infiltration in aneurysm walls. J Neurosurg. 2014;120:73–86.
Li ZF, Fang XG, Zhao R, Yang PF, Huang QH, Liu JM. Stromal cell-derived factor 1alpha facilitates aneurysm remodeling in elastase-induced rabbit saccular aneurysm. Cytokine. 2018;102:123–30.
Funding
The study was supported by the National Key R&D Program of China (2016YFC1300700) and Natural Science Foundation of China (no. 81771264).
Author information
Authors and Affiliations
Contributions
Y.Z.Y. and Q.H.H. contributed to project conception and design and provided financial support. Y.Z.Y., J.C.X., F.F.X., C.C.W, Z.W.Z., H.S.T., and Z.W.L conducted the experiment. Y.Z.Y., J.C.X., and F.F.X. participated in the collection of data, analyzed and interpreted data, and wrote the manuscript. Q.H.H. critically revised the article and approved the final version of the manuscript on behalf of all the authors. Y.Z.Y., J.C.X., and F.F.X. contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests. Guarantor: Qinghai Huang, M.D.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12975_2021_925_MOESM1_ESM.jpg
Supplementary file1. Determination of the most appropriate wall shear stress (WSS) stimulation and drug concentration. (A) In vitro co-culture schematic. Human umbilical venous endothelial cells (HUVECs) were plated on the bottom surface of the transwell membrane. After 2 h of culture, human brain vascular smooth muscle cells (HBVSMCs) were plated on the inner surface of the membrane. After 24 h of culture, the cells were dynamically cultured in the Flow Chamber. (B-E) Different WSS were applied to this co-culture model to identify the most suitable WSS application. The highest expression of SDF-1α, CXCR4, and MMP-2 was observed at12 dyn/cm2 WSS, and the expression of α-SMA was significantly lower than that of static culture. Therefore, WSS 12 dyn/cm2 was used for 12 h intervention. (F-I) Different concentrations of AMD3100 and SDF-1α were applied to the co-culture model to identify the most suitable drug concentration. At 12 dyn/cm2 WSS for 12 h, the highest expression of α-SMA was observed with 10 μM AMD3100, while the expression of MMP-2 was significantly inhibited. At 100 ng/ml SDF-1α, the expression of α-SMA was significantly inhibited, whereas the highest expression level of MMP-2 was observed at this concentration. Therefore, 10 μM AMD3100 and 100 ng/ml recombinant human SDF-1α were used for follow-up intervention. Each experiment was performed at least three times. * p < 0.05; ** p < 0.01; *** p < 0.001. (JPG 651 KB)
Rights and permissions
About this article
Cite this article
Yan, Y., Xiong, J., Xu, F. et al. SDF-1α/CXCR4 Pathway Mediates Hemodynamics-Induced Formation of Intracranial Aneurysm by Modulating the Phenotypic Transformation of Vascular Smooth Muscle Cells. Transl. Stroke Res. 13, 276–286 (2022). https://doi.org/10.1007/s12975-021-00925-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-021-00925-1