Abstract
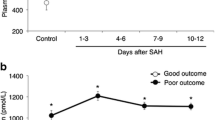
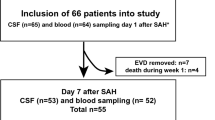
A matricellular protein osteopontin (OPN) is considered to exert neuroprotective and healing effects on neurovascular injuries in an acute phase of aneurysmal subarachnoid hemorrhage (SAH). However, the relationships between OPN expression and chronic shunt-dependent hydrocephalus (SDHC) have never been investigated. In 166 SAH patients (derivation and validation cohorts, 110 and 56, respectively), plasma OPN levels were serially measured at days1−3, 4−6, 7−9, and 10−12 after aneurysmal obliteration. The OPN levels and clinical factors were compared between patients with and without subsequent development of chronic SDHC. Plasma OPN levels in the SDHC patients increased from days 1−3 to days 4−6 and remained high thereafter, while those in the non-SDHC patients peaked at days 4−6 and then decreased over time. Plasma OPN levels had no correlation with serum levels of C-reactive protein (CRP), a systemic inflammatory marker. Univariate analyses showed that age, modified Fisher grade, acute hydrocephalus, cerebrospinal fluid drainage, and OPN and CRP levels at days 10−12 were significantly different between patients with and without SDHC. Multivariate analyses revealed that higher plasma OPN levels at days 10−12 were an independent factor associated with the development of SDHC, in addition to a more frequent use of cerebrospinal fluid drainage and higher modified Fisher grade at admission. Plasma OPN levels at days 10−12 maintained similar discrimination power in the validation cohort and had good calibration on the Hosmer-Lemeshow goodness-of-fit test. Prolonged higher expression of OPN may contribute to the development of post-SAH SDHC, possibly by excessive repairing effects promoting fibrosis in the subarachnoid space.


Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Xie Z, Hu X, Zan X, Lin S, Li H, You C. Predictors of shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage? A systematic review and meta-analysis. World Neurosurg. 2017;106:844–60.
Adil SM, Liu B, Charalambous LT, Kiyani M, Gramer R, Swisher CB, et al. Healthcare economics of hydrocephalus after aneurysmal subarachnoid hemorrhage in the United States. Transl Stroke Res. 2019;10:650–63.
Suzuki H, Muramatsu M, Tanaka K, Fujiwara H, Kojima T, Taki W. Cerebrospinal fluid ferritin in chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurol. 2006;253:1170–6.
Nakatsuka Y, Kawakita F, Yasuda R, Umeda Y, Toma N, Sakaida H, et al. Preventive effects of cilostazol against the development of shunt-dependent hydrocephalus after subarachnoid hemorrhage. J Neurosurg. 2017;127:319–26.
Chen S, Luo J, Reis C, Manaenko A, Zhang J. Hydrocephalus after subarachnoid hemorrhage: pathophysiology, diagnosis, and treatment. Biomed Res Int. 2017;2017:8584753. https://doi.org/10.1155/2017/8584753.
Suzuki H, Kinoshita N, Imanaka-Yoshida K, Yoshida T, Taki W. Cerebrospinal fluid tenascin-C increases preceding the development of chronic shunt-dependent hydrocephalus after subarachnoid hemorrhage. Stroke. 2008;39:1610–2.
Zhou Y, Yao Y, Sheng L, Zhang J, Zhang JH, Shao A. Osteopontin as a candidate of therapeutic application for the acute brain injury. J Cell Mol Med. 2020;24:8918–29.
Kawakita F, Kanamaru H, Asada R, Suzuki H. Potential roles of matricellular proteins in stroke. Exp Neurol. 2019;322:113057. https://doi.org/10.1016/j.expneurol.2019.113057.
Okamoto H, Imanaka-Yoshida K. Matricellular proteins: new molecular targets to prevent heart failure. Cardiovasc Ther. 2012;30:e198–209.
Nakatsuka Y, Shiba M, Nishikawa H, Terashima M, Kawakita F, Fujimoto M, et al. Acute-phase plasma osteopontin as an independent predictor for poor outcome after aneurysmal subarachnoid hemorrhage. Mol Neurobiol. 2018;55:6841–9.
Abate MG, Moretto L, Licari I, Esposito T, Capuano L, Olivieri C, et al. Osteopontin in the cerebrospinal fluid of patients with severe aneurysmal subarachnoid hemorrhage. Cells. 2019;8:695. https://doi.org/10.3390/cells8070695.
Suzuki H, Nishikawa H, Kawakita F. Matricellular proteins as possible biomarkers for early brain injury after aneurysmal subarachnoid hemorrhage. Neural Regen Res. 2018;13:1175–8.
Tsukui T, Ueha S, Abe J, Hashimoto SI, Shichino S, Shimaoka T, et al. Qualitative rather than quantitative changes are hallmarks of fibroblasts in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2013;183:758–73.
Dong J, Ma Q. Osteopontin enhances multi-walled carbon nanotube-triggered lung fibrosis by promoting TGF-β1 activation and myofibroblast differentiation. Part Fibre Toxicol. 2017;14:18. https://doi.org/10.1186/s12989-017-0198-0.
Suzuki H. Inflammation: a good research target to improve outcomes of poor-grade subarachnoid hemorrhage. Transl Stroke Res. 2019;10:597–600.
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery. 2006;59:21–6.
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5.
Germanwala AV, Huang J, Tamargo RJ. Hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:263–70.
Bae IS, Yi HJ, Choi KS, Chun HJ. Comparison of incidence and risk factors for shunt-dependent hydrocephalus in aneurysmal subarachnoid hemorrhage patients. J Cerebrovasc Endovasc Neurosurg. 2014;16:78–84.
Wilson CD, Safavi-Abbasi S, Sun H, Kalani MYS, Zhao YD, Levitt MR, et al. Meta-analysis and systematic review of risk factors for shunt dependency after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;126:586–95.
Erixon HO, Sorteberg A, Sorteberg W, Eide PK. Predictors of shunt dependency after aneurysmal subarachnoid hemorrhage: results of a single-center clinical trial. Acta Neurochir. 2014;156:2059–69.
Massicotte EM, Del Bigio MR. Human arachnoid villi response to subarachnoid hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg. 1999;91:80–4.
Motohashi O, Suzuki M, Shida N, Umezawa K, Ohtoh T, Sakurai Y, et al. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space: immunohistochemical study. Acta Neurochir. 1995;136:88–91.
Sheehan JP, Polin RS, Sheehan JM, Baskaya MK, Kassell NF. Factors associated with hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1999;45:1120–8.
Johanson CE, Szmydynger-Chodobska J, Chodobski A, Baird A, McMillan P, Stopa EG. Altered formation and bulk absorption of cerebrospinal fluid in FGF-2-induced hydrocephalus. Am J Phys. 1999;277:R263–71.
Kitazawa K, Tada T. Elevation of transforming growth factor-beta1 level in cerebrospinal fluid of patients with communicating hydrocephalus after subarachnoid hemorrhage. Stroke. 1994;25:1400–4.
Motohashi O, Suzuki M, Yanai N, Umezawa K, Shida N, Yoshimoto T. Thrombin and TGF-beta promote human leptomeningeal cell proliferation in vitro. Neurosci Lett. 1995;190:105–8.
Tada T, Kanaji M, Kobayashi S. Induction of communicating hydrocephalus in mice by intrathecal injection of human recombinant transforming growth factor-beta1. J Neuroimmunol. 1994;50:153–8.
Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010;29:295–307.
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Matricellular proteins osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61.
Suzuki H, Hasegawa Y, Chen W, Kanamaru K, Zhang JH. Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Ann Neurol. 2010;68:650–60.
Wu J, Zhang Y, Yang P, Enkhjargal B, Manaenko A, Tang J, et al. Recombinant osteopontin stabilizes smooth muscle cell phenotype via integrin receptor/integrin-linked kinase/Rac-1 pathway after subarachnoid hemorrhage in rats. Stroke. 2016;47:1319–27.
Enkhjargal B, McBride DW, Manaenko A, Reis C, Sakai Y, Tang J, et al. Intranasal administration of vitamin D attenuates blood–brain barrier disruption through endogenous upregulation of osteopontin and activation of CD44/P-gp glycosylation signaling after subarachnoid hemorrhage in rats. J Cereb Blood Flow Metab. 2017;37:2555–66.
Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010;41:1783–90.
Sun CM, Enkhjargal B, Reis C, Zhou KR, Xie ZY, Wu LY, et al. Osteopontin attenuates early brain injury through regulating autophagy-apoptosis interaction after subarachnoid hemorrhage in rats. CNS Neurosci Ther. 2019;25:1162–72.
He J, Liu M, Liu Z, Luo L. Recombinant osteopontin attenuates experimental cerebral vasospasm following subarachnoid hemorrhage in rats through an anti-apoptotic mechanism. Brain Res. 1611;2015:74–83.
Sun C, Enkhjargal B, Reis C, Zhang T, Zhu Q, Zhou K, et al. Osteopontin-enhanced autophagy attenuates early brain injury via FAK–ERK pathway and improves long-term outcome after subarachnoid hemorrhage in rats. Cells. 2019;8:980. https://doi.org/10.3390/cells8090980.
Abdelaziz Mohamed I, Gadeau AP, Hasan A, Abdulrahman N, Mraiche F. Osteopontin: a promising therapeutic target in cardiac fibrosis. Cells. 2019;8:1558. https://doi.org/10.3390/cells8121558.
Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell Commun Signal. 2009;3:311–22.
Lin R, Wu S, Zhu D, Qin M, Liu X. Osteopontin induces atrial fibrosis by activating Akt/GSK-3β/β-catenin pathway and suppressing autophagy. Life Sci. 2020;245:117328. https://doi.org/10.1016/j.lfs.2020.117328.
Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP, et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol. 2012;71:743–52.
Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870–86.
Gliem M, Krammes K, Liaw L, van Rooijen N, Hartung HP, Jander S. Macrophage-derived osteopontin induces reactive astrocyte polarization and promotes re-establishment of the blood brain barrier after ischemic stroke. Glia. 2015;63:2198–207.
Ueno M, Chiba Y, Matsumoto K, Murakami R, Fujihara R, Kawauchi M, et al. Blood-brain barrier damage in vascular dementia. Neuropathology. 2016;36:115–24.
Acknowledgments
We thank Ms. Chiduru Nakamura (Department of Neurosurgery, Mie University Graduate School of Medicine) for her technical assistance.
Funding
This work was supported by JSPS KAKENHI Grant Number JP17K10825 and JP20K09346 to Dr. Hidenori Suzuki.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethical committee of Mie University Hospital (Ethics approval number: 2544 and H2018-031).
Informed Consent
The ethical committee waived the need for informed consent with opt-out.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
pSEED group members along with their affiliations listed in Online Resource.
Supplementary Information
ESM 1
(PDF 173 kb)
Rights and permissions
About this article
Cite this article
Asada, R., Nakatsuka, Y., Kanamaru, H. et al. Higher Plasma Osteopontin Concentrations Associated with Subsequent Development of Chronic Shunt-Dependent Hydrocephalus After Aneurysmal Subarachnoid Hemorrhage. Transl. Stroke Res. 12, 808–816 (2021). https://doi.org/10.1007/s12975-020-00886-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-020-00886-x