Abstract
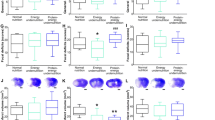
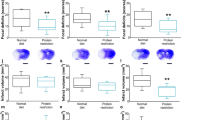
Protein-energy malnutrition (PEM) pre-existing at stroke onset is believed to worsen functional outcome, yet the underlying mechanisms are not fully understood. Since brain inflammation is an important modulator of neurological recovery after stroke, we explored the impact of PEM on neuroinflammation in the acute period in relation to stroke-initiated sensori-motor abnormalities. Adult rats were fed a low-protein (LP) or normal protein (NP) diet for 28 days before inducing photothrombotic stroke (St) in the forelimb region of the motor cortex or sham surgery; the diets continued for 3 days after the stroke. Protein-energy status was assessed by a combination of body weight, food intake, serum acute phase proteins and corticosterone, and liver lipid content. Deficits in motor function were evaluated in the horizontal ladder walking and cylinder tasks at 3 days after stroke. The glial response and brain elemental signature were investigated by immunohistochemistry and micro-X-ray fluorescence imaging, respectively. The LP-fed rats reduced food intake, resulting in PEM. Pre-existing PEM augmented stroke-induced abnormalities in forelimb placement accuracy on the ladder; LP-St rats made more errors (29 ± 8%) than the NP-St rats (15 ± 3%; P < 0.05). This was accompanied by attenuated astrogliosis in the peri-infarct area by 18% and reduced microglia activation by up to 41 and 21% in the peri-infarct area and the infarct rim, respectively (P < 0.05). The LP diet altered the cortical Zn, Ca, and Cl signatures (P < 0.05). Our data suggest that proactive treatment of pre-existing PEM could be essential for optimal post-stroke recovery.






Similar content being viewed by others
References
Foley N, Teasell R, Richardson M, Bhogal S, J. S. Nutritional interventions following stroke: evidence-based review of stroke rehabilitation (EBRSR). http://www.ebrsr.com/evidence-review/16-nutritional-interventions-following-stroke. September 2016 Accessed February 23, 2017.
Davis JP, Wong AA, Schluter PJ, Henderson RD, O'Sullivan JD, Read SJ. Impact of premorbid undernutrition on outcome in stroke patients. Stroke. 2004;35(8):1930–4. https://doi.org/10.1161/01.STR.0000135227.10451.c9.
Martineau J, Bauer JD, Isenring E, Cohen S. Malnutrition determined by the patient-generated subjective global assessment is associated with poor outcomes in acute stroke patients. Clin Nutr. 2005;24(6):1073–7. https://doi.org/10.1016/j.clnu.2005.08.010.
Yoo SH, Kim JS, Kwon SU, Yun SC, Koh JY, Kang DW. Undernutrition as a predictor of poor clinical outcomes in acute ischemic stroke patients. Arch Neurol. 2008;65(1):39–43. https://doi.org/10.1001/archneurol.2007.12.
Gomes F, Emery PW, Weekes CE. Risk of malnutrition is an independent predictor of mortality, length of hospital stay, and hospitalization costs in stroke patients. J Stroke Cerebrovasc Dis. 2016;25(4):799–806. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.12.017.
Food Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial. Stroke. 2003;34(6):1450–6. https://doi.org/10.1161/01.STR.0000074037.49197.8C.
Kokura Y, Maeda K, Wakabayashi H, Nishioka S, Higashi S. High nutritional-related risk on admission predicts less improvement of functional independence measure in geriatric stroke patients: a retrospective cohort study. J Stroke Cerebrovasc Dis. 2016;25(6):1335–41. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.01.048.
Nii M, Maeda K, Wakabayashi H, Nishioka S, Tanaka A. Nutritional improvement and energy intake are associated with functional recovery in patients after cerebrovascular disorders. J Stroke Cerebrovasc Dis. 2016;25(1):57–62. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.08.033.
Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. https://doi.org/10.1038/nm.2399.
Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, et al. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014;62(12):2022–33. https://doi.org/10.1002/glia.22723.
Patel AR, Ritzel R, Mccullough LD, Liu F. Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol. 2013;5(2):73–90.
Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–34. https://doi.org/10.1002/glia.20207.
Denes A, Ferenczi S, Kovacs KJ. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood-brain barrier damage and brain oedema independently of infarct size. J Neuroinflammation. 2011;8(1):164. https://doi.org/10.1186/1742-2094-8-164.
Langdon KD, Maclellan CL, Corbett D. Prolonged, 24-h delayed peripheral inflammation increases short- and long-term functional impairment and histopathological damage after focal ischemia in the rat. J Cereb Blood Flow Metab. 2010;30(8):1450–9. https://doi.org/10.1038/jcbfm.2010.23.
Bobyn PJ, Corbett D, Saucier DM, Noyan-Ashraf MH, Juurlink BH, Paterson PG. Protein-energy malnutrition impairs functional outcome in global ischemia. Exp Neurol. 2005;196(2):308–15. https://doi.org/10.1016/j.expneurol.2005.08.006.
Ji L, Nazarali AJ, Paterson PG. Protein-energy malnutrition increases activation of the transcription factor, nuclear factor kappaB, in the gerbil hippocampus following global ischemia. J Nutr Biochem. 2008;19(11):770–7. https://doi.org/10.1016/j.jnutbio.2007.09.011.
Smith SE, Prosser-Loose EJ, Colbourne F, Paterson PG. Protein-energy malnutrition alters thermoregulatory homeostasis and the response to brain ischemia. Curr Neurovasc Res. 2011;8(1):64–74. https://doi.org/10.2174/156720211794520206.
Yaqoob P, Calder PC. Nutrition in immune function. In: Zempleni J, Daniel H, editors. Molecular nutrition. Cambridge: CABI Publishing; 2003. p. 349–67.
Young W, Rappaport ZH, Chalif DJ, Flamm ES. Regional brain sodium, potassium, and water changes in the rat middle cerebral artery occlusion model of ischemia. Stroke. 1987;18(4):751–9. https://doi.org/10.1161/01.STR.18.4.751.
Caine S, Hackett MJ, Hou H, Kumar S, Maley J, Ivanishvili Z, et al. A novel multi-modal platform to image molecular and elemental alterations in ischemic stroke. Neurobiol Dis. 2016;91:132–42. https://doi.org/10.1016/j.nbd.2016.03.006.
Baier S, Kramer P, Grudzenski S, Fatar M, Kirsch S, Schad LR. Chlorine and sodium chemical shift imaging during acute stroke in a rat model at 9.4 Tesla. Magn Reson Mater Phys. 2014;27(1):71–9. https://doi.org/10.1007/s10334-013-0398-z.
Nilsson P, Laursen H, Hillered L, Hansen AJ. Calcium movements in traumatic brain injury: the role of glutamate receptor-operated ion channels. J Cerebr Blood Flow Metab. 1996;16(2):262–70. https://doi.org/10.1097/00004647-199603000-00011.
Annunziato L, Boscia F, Pignataro G. Ionic transporter activity in astrocytes, microglia, and oligodendrocytes during brain ischemia. J Cereb Blood Flow Metab. 2013;33(7):969–82. https://doi.org/10.1038/jcbfm.2013.44.
Galasso SL, Dyck RH. The role of zinc in cerebral ischemia. Mol Med. 2007;13(7–8):380–7. https://doi.org/10.2119/2007-00044.Galasso.
Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Mueller RN, Zeng Y, et al. Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp Neurol. 2006;198(2):285–93. https://doi.org/10.1016/j.expneurol.2005.08.030.
Alaverdashvili M, Li X, Paterson PG. Protein-energy malnutrition causes deficits in motor function in adult male rats. J Nutr. 2015;145(11):2503–11. https://doi.org/10.3945/jn.115.216382.
Alaverdashvili M, Paterson PG, Bradley MP. Laser system refinements to reduce variability in infarct size in the rat photothrombotic stroke model. J Neurosci Methods. 2015;247:58–66. https://doi.org/10.1016/j.jneumeth.2015.03.029.
Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115(2):169–79. https://doi.org/10.1016/S0165-0270(02)00012-2.
Schallert T, Woodlee M. Orienting and placing. In: Whishaw IQ, Kolb B, editors. The behavior of the laboratory rat. New York: Oxford University Press; 2005. p. 129–40.
Alaverdashvili M, Hackett MJ, Pickering IJ, Paterson PG. Laminar-specific distribution of zinc: evidence for presence of layer IV in forelimb motor cortex in the rat. NeuroImage. 2014;103:502–10. https://doi.org/10.1016/j.neuroimage.2014.08.046.
Nagai N, Kawao N, Okada K, Ishida C, Okumoto K, Ueshima S, et al. Initial brain lesion size affects the extent of subsequent pathophysiological responses. Brain Res. 2010;1322:109–17. https://doi.org/10.1016/j.brainres.2010.01.077.
Alaverdashvili M, Hackett MJ, Caine S, Paterson PG. Parallel changes in cortical neuron biochemistry and motor function in protein-energy malnourished adult rats. NeuroImage. 2017;149:275–84. https://doi.org/10.1016/j.neuroimage.2017.02.010.
Schroeter M, Jander S, Huitinga I, Witte OW, Stoll G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke. 1997;28(2):382–6. https://doi.org/10.1161/01.STR.28.2.382.
Peters L, O'Connor C, Giroux I, Teasell R, Foley N. Screening and assessment of nutritional status following stroke: results from a national survey of registered dietitians in Canada. Disabil Rehabil. 2015;37(26):1–5. https://doi.org/10.3109/09638288.2015.1027006.
Schroeter M, Franke C, Stoll G, Hoehn M. Dynamic changes of magnetic resonance imaging abnormalities in relation to inflammation and glial responses after photothrombotic cerebral infarction in the rat brain. Acta Neuropathol. 2001;101(2):114–22.
Nowicka D, Rogozinska K, Aleksy M, Witte OW, Skangiel-Kramska J. Spatiotemporal dynamics of astroglial and microglial responses after photothrombotic stroke in the rat brain. Acta Neurobiol Exp (Wars). 2008;68(2):155–68.
Amantea D, Tassorelli C, Petrelli F, Certo M, Bezzi P, Micieli G, et al. Understanding the multifaceted role of inflammatory mediators in ischemic stroke. Curr Med Chem. 2014;21(18):2098–117. https://doi.org/10.2174/0929867321666131227162634.
Petito CK, Morgello S, Felix JC, Lesser ML. The two patterns of reactive astrocytosis in postischemic rat brain. J Cereb Blood Flow Metab. 1990;10(6):850–9. https://doi.org/10.1038/jcbfm.1990.141.
Schiffer D, Giordana MT, Migheli A, Giaccone G, Pezzotta S, Mauro A. Glial fibrillary acidic protein and vimentin in the experimental glial reaction of the rat brain. Brain Res. 1986;374(1):110–8. https://doi.org/10.1016/0006-8993(86)90399-9.
Giulian D, Corpuz M, Chapman S, Mansouri M, Robertson C. Reactive mononuclear phagocytes release neurotoxins after ischemic and traumatic injury to the central nervous system. J Neurosci Res. 1993;36(6):681–93. https://doi.org/10.1002/jnr.490360609.
Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8(1):174. https://doi.org/10.1186/1742-2094-8-174.
Takahashi S, Hatashita S, Taba Y, Sun XZ, Kubota Y, Yoshida S. Determination of the spatial distribution of major elements in the rat brain with X-ray fluorescence analysis. J Neurosci Methods. 2000;100(1–2):53–62. https://doi.org/10.1016/S0165-0270(00)00231-4.
Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13(4):661–70. https://doi.org/10.1007/s13311-016-0483-x.
Lowry SF, Coyle S. Hypercatabolic states. In: Ross AC, Caballero B, Cousins R, Tucker K, Ziegler R, editors. Modern nutrition in health and disease. Philadelphia: Wolters KluwerHealth / Lippincott Williams and Wilkins; 2014. p. 1261–72.
Acknowledgements
The authors thank Sonia Cyrenne, Angela Cooper, and Megan Morgan for technical assistance and Samuel M. Webb and Courtney Roach for experimental station setup at Stanford Synchrotron Radiation Lightsource (SSRL) and providing thoughtful comments on the data set.
Sources of Funding
This study was funded by the Canadian Institutes of Health Research (CIHR)/Heart and Stroke Foundation of Canada (HSF) Grant No. 99472 (P.G.P. and H.N.); CIHR Operating Grant No. 111124 (P.G.P.); and HSF Grant-In-Aid (P.G.P).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Disclosures
Use of the SSRL, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS) (including P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Electronic Supplementary Material
ESM 1
(DOCX 10.3 mb)
Rights and permissions
About this article
Cite this article
Alaverdashvili, M., Caine, S., Li, X. et al. Protein-Energy Malnutrition Exacerbates Stroke-Induced Forelimb Abnormalities and Dampens Neuroinflammation. Transl. Stroke Res. 9, 622–630 (2018). https://doi.org/10.1007/s12975-018-0613-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0613-3