Abstract
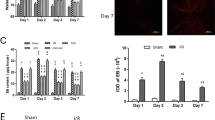
Thrombin mediates the life-threatening cerebral edema and blood–brain barrier (BBB) damage that occurs after intracerebral hemorrhage (ICH). We previously found that the selective cannabinoid receptor 2 (CB2R) agonist JWH-133 reduced brain edema and neurological deficits following germinal matrix hemorrhage (GMH). We explored whether CB2R stimulation ameliorated thrombin-induced brain edema and BBB permeability as well as the possible molecular mechanism involved. A total of 144 Sprague–Dawley (S-D) rats received a thrombin (20 U) injection in the right basal ganglia. JWH-133 (1.5 mg/kg) or SR-144528 (3.0 mg/kg) and vehicle were intraperitoneally (i.p.) injected 1 h after surgery. Brain water content measurement, Evans blue (EB) extravasation, Western blot, and immunofluorescence were used to study the effects of a CB2R agonist 24 h after surgery. The results demonstrated that JWH-133 administration significantly decreased thrombin-induced brain edema and reduced the number of Iba-1-positive microglia. JWH-133 also decreased the number of P44/P42(+)/Iba-1(+) microglia, lowered Evans blue extravasation, and inhibited the elevated matrix metallopeptidase (MMP)-9 and matrix metallopeptidase (MMP)-12 activities. However, a selective CB2R antagonist (SR-144528) reversed these effects. We demonstrated that CB2R stimulation reduced thrombin-induced brain edema and alleviated BBB damage. We also found that matrix metalloproteinase suppression may be partially involved in these processes.






Similar content being viewed by others
References
Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat Rev Neurol. 2010;6(11):593–601. doi:10.1038/nrneurol.2010.146.
Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–31. doi:10.1016/s1474-4422(12)70104-7.
Bodmer D, Vaughan KA, Zacharia BE, Hickman ZL, Connolly ES. The molecular mechanisms that promote edema after intracerebral hemorrhage. Transl Stroke Res. 2012;3 Suppl 1:52–61. doi:10.1007/s12975-012-0162-0.
Yang GY, Chen SF, Kinouchi H, Chan PH, Weinstein PR. Edema, cation content, and ATPase activity after middle cerebral artery occlusion in rats. Stroke; J Cereb Circ. 1992;23(9):1331–6.
Freeman WD, Barrett KM, Bestic JM, Meschia JF, Broderick DF, Brott TG. Computer-assisted volumetric analysis compared with ABC/2 method for assessing warfarin-related intracranial hemorrhage volumes. Neurocrit Care. 2008;9(3):307–12. doi:10.1007/s12028-008-9089-4.
Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke; J Cereb Circ. 2007;38(2 Suppl):759–62. doi:10.1161/01.STR.0000247868.97078.10.
Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, et al. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke; J Cereb Circ. 1998;29(12):2580–6.
Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg. 1996;84(1):91–6. doi:10.3171/jns.1996.84.1.0091.
Kitaoka T, Hua Y, Xi G, Hoff JT, Keep RF. Delayed argatroban treatment reduces edema in a rat model of intracerebral hemorrhage. Stroke; J Cereb Circ. 2002;33(12):3012–8. doi:10.1161/01.str.0000037673.17260.1b.
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke; J Cereb Circ. 2002;33(10):2478–84.
Guan JX, Sun SG, Cao XB, Chen ZB, Tong ET. Effect of thrombin on blood brain barrier permeability and its mechanism. Chin Med J. 2004;117(11):1677–81.
Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem. 2003;84(1):3–9.
Xi G, Hua Y, Keep RF, Duong HK, Hoff JT. Activation of p44/42 mitogen activated protein kinases in thrombin-induced brain tolerance. Brain Res. 2001;895(1–2):153–9.
Kreitzer FR, Stella N. The therapeutic potential of novel cannabinoid receptors. Pharmacol Ther. 2009;122(2):83–96. doi:10.1016/j.pharmthera.2009.01.005.
Devane WA, Dysarz 3rd FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605–13.
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–4. doi:10.1038/346561a0.
Hillard CJ. Role of cannabinoids and endocannabinoids in cerebral ischemia. Curr Pharm Des. 2008;14(23):2347–61.
Ramirez SH, Hasko J, Skuba A, Fan S, Dykstra H, McCormick R, et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood–brain barrier dysfunction under inflammatory conditions. J Neurosci: Off J Soc Neurosci. 2012;32(12):4004–16. doi:10.1523/JNEUROSCI.4628-11.2012.
Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. Br J Pharmacol. 2008;153(2):240–51. doi:10.1038/sj.bjp.0707584.
Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Exp Rev Mole Med. 2009;11, e3. doi:10.1017/S1462399409000957.
Tao Y, Tang J, Chen Q, Guo J, Li L, Yang L, et al. Cannabinoid CB2 receptor stimulation attenuates brain edema and neurological deficits in a germinal matrix hemorrhage rat model. Brain Res. 2015;1602:127–35. doi:10.1016/j.brainres.2015.01.025.
Romanic AM, Madri JA. Extracellular matrix-degrading proteinases in the nervous system. Brain Pathol. 1994;4(2):145–56.
Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2(7):502–11. doi:10.1038/35081571.
Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013;4(3):279–85. doi:10.1007/s12975-012-0209-2.
Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–91. doi:10.1038/nature11556.
Jiang Y, Wu J, Hua Y, Keep RF, Xiang J, Hoff JT, et al. Thrombin-receptor activation and thrombin-induced brain tolerance. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2002;22(4):404–10. doi:10.1097/00004647-200204000-00004.
Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, Garcia-Gutierrez MS, Vivancos J, Nombela F, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke; J Cereb Circ. 2012;43(1):211–9. doi:10.1161/STROKEAHA.111.631044.
Chen Y, Zhang Y, Tang J, Liu F, Hu Q, Luo C, et al. Norrin protected blood–brain barrier via frizzled-4/beta-catenin pathway after subarachnoid hemorrhage in rats. Stroke; J Cereb Circ. 2015;46(2):529–36. doi:10.1161/STROKEAHA.114.007265.
Tang JH, Yan FH, Zhou ML, Xu PJ, Zhou J, Fan J. Evaluation of computer-assisted quantitative volumetric analysis for pre-operative resectability assessment of huge hepatocellular carcinoma. Asian Pac J Cancer Prev : APJCP. 2013;14(5):3045–50.
Hua Y, Keep RF, Hoff JT, Xi G. Thrombin preconditioning attenuates brain edema induced by erythrocytes and iron. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2003;23(12):1448–54. doi:10.1097/01.WCB.0000090621.86921.D5.
Xi G, Keep RF, Hua Y, Xiang J, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke; J Cereb Circ. 1999;30(6):1247–55.
Liu DZ, Ander BP, Xu H, Shen Y, Kaur P, Deng W, et al. Blood–brain barrier breakdown and repair by Src after thrombin-induced injury. Ann Neurol. 2010;67(4):526–33. doi:10.1002/ana.21924.
Lai YL, Smith PM, Lamm WJ, Hildebrandt J. Sampling and analysis of cerebrospinal fluid for chronic studies in awake rats. J Appl Physiol Respir Environ Exerc Physiol. 1983;54(6):1754–7.
Thiel A, Heiss WD. Imaging of microglia activation in stroke. Stroke; J Cereb Circ. 2011;42(2):507–12. doi:10.1161/STROKEAHA.110.598821.
Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi:10.1126/science.1110647.
Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood–brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke; J Cereb Circ. 2006;37(4):1087–93. doi:10.1161/01.STR.0000206281.77178.ac.
Fujimoto S, Katsuki H, Ohnishi M, Takagi M, Kume T, Akaike A. Thrombin induces striatal neurotoxicity depending on mitogen-activated protein kinase pathways in vivo. Neuroscience. 2007;144(2):694–701. doi:10.1016/j.neuroscience.2006.09.049.
Fujimoto S, Katsuki H, Kume T, Akaike A. Thrombin-induced delayed injury involves multiple and distinct signaling pathways in the cerebral cortex and the striatum in organotypic slice cultures. Neurobiol Dis. 2006;22(1):130–42. doi:10.1016/j.nbd.2005.10.008.
Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901. doi:10.1152/physrev.00035.2003.
Bauer AT, Burgers HF, Rabie T, Marti HH. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2010;30(4):837–48. doi:10.1038/jcbfm.2009.248.
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2007;27(4):697–709. doi:10.1038/sj.jcbfm.9600375.
Hamann GF, Burggraf D, Martens HK, Liebetrau M, Jager G, Wunderlich N, et al. Mild to moderate hypothermia prevents microvascular basal lamina antigen loss in experimental focal cerebral ischemia. Stroke; a journal of cerebral circulation. 2004;35(3):764–9. doi:10.1161/01.STR.0000116866.60794.21.
Abilleira S, Montaner J, Molina CA, Monasterio J, Castillo J, Alvarez-Sabin J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003;99(1):65–70. doi:10.3171/jns.2003.99.1.0065.
Castellazzi M, Tamborino C, De Santis G, Garofano F, Lupato A, Ramponi V, et al. Timing of serum active MMP-9 and MMP-2 levels in acute and subacute phases after spontaneous intracerebral hemorrhage. Acta Neurochir Suppl. 2010;106:137–40. doi:10.1007/978-3-211-98811-4_24.
Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12(4):441–5. doi:10.1038/nm1387.
Power C, Henry S, Del Bigio MR, Larsen PH, Corbett D, Imai Y, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53(6):731–42. doi:10.1002/ana.10553.
del Zoppo GJ, Frankowski H, Gu YH, Osada T, Kanazawa M, Milner R, et al. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2012;32(5):919–32. doi:10.1038/jcbfm.2012.11.
Maddahi A, Ansar S, Chen Q, Edvinsson L. Blockade of the MEK/ERK pathway with a raf inhibitor prevents activation of pro-inflammatory mediators in cerebral arteries and reduction in cerebral blood flow after subarachnoid hemorrhage in a rat model. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2011;31(1):144–54. doi:10.1038/jcbfm.2010.62.
Adhikary S, Kocieda VP, Yen JH, Tuma RF, Ganea D. Signaling through cannabinoid receptor 2 suppresses murine dendritic cell migration by inhibiting matrix metalloproteinase 9 expression. Blood. 2012;120(18):3741–9. doi:10.1182/blood-2012-06-435362.
Acknowledgments
We would like to thank Dr. Ya Hua from the University of Michigan for her professional comments on this research. This work was supported by grants 81571130 (Z.C) and 81070929 (Z.C) from the National Natural Science Foundation of China and 2014CB541606 (H.F) from the National Key Basic Research Development Program (973 Program) of China.
Author Contributions
ZC made substantial contributions to the conception and design. LL and YHT performed the experiments and acquired the data. JT and QWC measured the ventricular volume and cortical length. YJC and YYF participated in tissue fixation and immunohistochemistry. YY and LMY were responsible for supervising all experiments, data analysis and drafting of the manuscript. HF and GZ read and revised some parts of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
Lin Li, Yihao Tao, Jun Tang, Qianwei Chen, Yang Yang, Zhou Feng, Yujie Chen, Li Ming Yang, Yunfeng Yang, Hua Feng, and Zhi Chen declare that they have no conflicts of interest.
Compliance with Ethics Requirements
All institutional and national guidelines for the care and use of laboratory animals were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lin Li and Yihao Tao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, L., Tao, Y., Tang, J. et al. A Cannabinoid Receptor 2 Agonist Prevents Thrombin-Induced Blood–Brain Barrier Damage via the Inhibition of Microglial Activation and Matrix Metalloproteinase Expression in Rats. Transl. Stroke Res. 6, 467–477 (2015). https://doi.org/10.1007/s12975-015-0425-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-015-0425-7