Abstract
There were no data comparing the in-hospital outcomes after transcatheter aortic valve implantation (TAVI) with those after surgical aortic valve replacement (SAVR) in Japan. Among consecutive patients with severe AS between April 2018 and December 2020 in the CURRENT AS Registry-2, we identified 1714 patients who underwent aortic valve replacement (TAVI group: 1134 patients, and SAVR group: 580 patients). Patients in the TAVI group were much older (84.4 versus 73.6 years, P < 0.001) and more often had comorbidities than those in the SAVR group. In-hospital death rate was numerically lower in the TAVI group than in the SAVR group (0.6% versus 2.2%). After excluding patients with dialysis, in-hospital death rate was very low and comparable in the TAVI and SAVR groups (0.6% versus 0.8%). The rates of major bleeding and new-onset atrial fibrillation during index hospitalization were higher after SAVR than after TAVI (72% versus 20%, and 26% versus 4.6%, respectively), while the rate of pacemaker implantation was higher after TAVI than after SAVR (8.1% versus 2.4%). Regarding the echocardiographic data at discharge, the prevalence of patient-prosthesis mismatch was lower in the TAVI group than in the SAVR group (moderate: 9.0% versus 26%, and severe: 2.6% versus 4.8%). In this real-world data in Japan, TAVI compared with SAVR was chosen in much older patients with more comorbidities with severe AS. In-hospital death rate was numerically lower in the TAVI group than in the SAVR group.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Aortic valve replacement (AVR) is the only way to improve clinical outcomes in patients with severe aortic stenosis (AS). In the current era, transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) are the interventional options for patients with severe AS and have been reported to improve clinical outcomes compared to conservative therapy [1]. Several randomized controlled trials (RCTs) demonstrated comparable outcomes between SAVR and TAVI in patients with high to low surgical risk [2,3,4,5,6]. Based on these favorable outcomes of patients who underwent TAVI, the number of TAVI procedures has increased worldwide and the difference in age between SAVR and TAVI patients has decreased [7, 8]. However, there is a scarcity of data evaluating the practice pattern in the selection of treatment strategies for severe AS (TAVI, SAVR, or conservative), and comparing in-hospital outcomes between TAVI and SAVR in contemporary daily clinical practice [9]. Therefore, the aim of this study was to evaluate the patient characteristics and in-hospital outcomes in patients who underwent TAVI or SAVR in a large real-world Japanese registry of consecutive patients with severe AS in the current TAVI era.
Methods
Study population
Contemporary outcomes after sURgery and medical tREatmeNT in patients with severe Aortic Stenosis (CURRENT AS) registry-2 is a prospective, physician-initiated, non-company sponsored, multi-center registry enrolling consecutive patients who were diagnosed as having severe AS between April 2018 and December 2020 among 21 participating centers in Japan (Supplemental Appendix A). There were 10 centers where both SAVR and TAVI were available, 10 centers where only SAVR was available, and 1 center where neither SAVR nor TAVI was available. Inclusion and exclusion criteria of the CURRENT AS Registry-2 were described previously and were identical to those in the CURRENT AS Registry-1 [10, 11]. We defined patients with severe AS in this study as those who met at least one of the 3 echocardiographic criteria (peak aortic jet velocity [Vmax] > 4.0 m/s, mean aortic pressure gradient [PG] > 40 mmHg, or aortic valve area [AVA] < 1.0 cm2) for the first time during the enrollment period.
Initial treatment strategies (initial AVR or conservative) were determined by the discussion between the attending physician and the patients with occasional heart-team consultation. The choice of initial SAVR strategy or initial TAVI strategy was made based on the consensus in the heart team and the preference of the patients. Among 3369 patients enrolled in the CURRENT AS Registry-2, the current study population consisted of 1742 patients (51.7%) in whom initial AVR strategy was selected (initial TAVI strategy: 1148 patients [34.0%], and initial SAVR strategy: 594 patients [17.6%]) after excluding 1628 patients (48.3%) in whom conservative strategy was selected. Finally, 1714 patients actually underwent AVR (TAVI group: 1134 patients [66.1%], and SAVR group: 580 patients [33.9%]) (Fig. 1). The Number of cases in each participating center was described in Supplemental table 1.
The follow-up was commenced on the day of AVR. The protocol was approved by the institutional review boards in all 21 participating centers. Written informed consent was obtained from each patient in 19 centers, while in the remaining 2 centers, the opt-out strategy waiving written informed consent was adopted with permission by the institutional review boards.
Data collection and clinical outcomes
Details of data collection and definitions were described previously [11]. For the current analysis, the primary outcome measure was in-hospital death. The secondary outcome measures were stroke, disabling stroke, major bleeding, newly diagnosed atrial fibrillation (AF), pacemaker implantation, acute kidney injury, major vascular complication, intensive care unit stay after the procedure, hospital stay after the procedure, and hemodynamic parameters by echocardiography. In this study, we only evaluated those events, which occurred during or after AVR and up to hospital discharge. The definitions of clinical outcomes were described in Supplemental Appendix B. Clinical event committee adjudicated death, symptomatic stroke, and major bleeding (Supplemental Appendix C).
Statistical analysis
We compared the patient characteristics, echocardiographic characteristics, procedural parameters, and in-hospital outcomes between the SAVR and TAVI groups. Moreover, patients’ characteristics were evaluated according to the 3 age categories (< 75 years, ≥ 75 years and < 80 years, and ≥ 80 years). Regarding clinical outcomes, we conducted a sub-group analysis stratified by the presence of dialysis, because TAVI for patients with dialysis was not approved in Japan during the enrollment period of the present study. We also conducted a sub-group analysis stratified by the presence of concomitant procedure, bicuspid aortic valve (BAV), coronary artery disease (CAD), any valvular disease, and STS score categories.
We presented categorical variables as numbers and percentages and continuous variables as mean ± standard deviation or median with interquartile range (IQR). We compared patient characteristics using the χ2 test for categorical variables and Student’s t-test or Wilcoxon rank-sum test for continuous variables based on their distributions. Because the selection of SAVR and TAVI was based on not only the patient characterisitics, but also other factors, the selection bias was inevitable. Therefore, we reported the in-hospital events only numerically as number and percentages without hypothesis testing. In addition, we did not construct the multivariable models because of the small number of events for the large differences in many patient characteristics. A two-sided P value < 0.05 was regarded as statistically significant for all tests. All analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
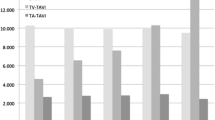
Patients in the TAVI group were much older than those in the SAVR group (mean age: 84.4 years versus 73.6 years, P < 0.001) (Table 1, Supplemental Table 2). Patients with ≥ 80 years of age were predominantly treated with TAVI, while patients with < 80 years of age were more often treated by SAVR (Fig. 2). In the SAVR group, 52% of patients were men, while in the TAVI group, only 35% of patients were men. Society of Thoracic Surgeons-Predicted Risk Of Mortality (STS-PROM) score was higher in the TAVI group than in the SAVR group (4.6% versus 2.9%, P < 0.001). Patients in the TAVI group more often had comorbidities such as prior coronary artery bypass grafting (CABG), end-stage renal disease not on dialysis, anemia, moderate to severe chronic lung disease, and malignancy currently under treatment than patients in the SAVR group. Clinical frailty scale was much higher in the TAVI group than in the SAVR group. Nevertheless, patients with dialysis were almost exclusively treated by SAVR (Table 1, Supplemental table 2).
The etiology of AS was degenerative in 96% of patients in the TAVI group, while it was congenital in 22% of patients in the SAVR group. More patients in the TAVI group had symptoms probably related to AS than those in the SAVR group.
Regarding the echocardiographic variables, severity of AS and flow pattern were not different between the SAVR and TAVI groups. Any combined valvular disease was more prevalent in the SAVR group than in the TAVI group (28% versus 23%, P = 0.02). Nevertheless, the prevalence of moderate or severe mitral regurgitation was comparable between the 2 groups (11% versus 11%, P = 0.78) (Supplemental Table 3).
In terms of patient characteristics categorized by age, among patients younger than 75 years, those in the TAVI group were older, frailer, and more frequently presented with comorbidities such as prior CABG, symptomatic stroke, anemia, malignancy, immunosuppressive therapy, and moderate or severe chronic lung disease compared to patients in the SAVR group (Supplemental table 4).
Regarding patient characteristics in the SAVR group dividing with/without dialysis, patients with dialysis exhibited a higher prevalence of comorbidities including prior symptomatic stroke, atrial fibrillation or flutter, anemia, aortic and/or peripheral vascular disease, and coronary artery disease. Furthermore, patients with dialysis were more frail and had higher surgical risk scores compared to those without dialysis (Supplemental table 5).
Procedural characteristics
In the SAVR group, 48% of patients underwent concomitant procedures such as CABG (28%), mitral valve surgery (9.5%), replacement of ascending aorta (8.4%), Maze procedure (7.4%), and tricuspid valve surgery (5.5%). Bioprosthetic valve was used in 96% of patients. The most frequently used bioprosthetic valve size was 21 mm followed by 23 mm valve (Supplemental table 6).
In the TAVI group, PCI before TAVI was conducted in a separate session from TAVI in 6.0% of patients and in the same session with TAVI in 0.9% of patients. TAVI procedure was performed under local anesthesia with conscious sedation in 61% of patients, and through transfemoral approach in 92% of patients. Balloon-expandable valve (SAPIEN 3; Edwards Lifesciences, CA, USA) was used in 69% of patients. The most frequently used valve size was 23 mm followed by 26 mm valve for the balloon-expandable valve, and 26 mm followed by 29 mm valve for the self-expandable valve (Supplemental table 6).
In-hospital outcomes
Intensive care unit stay after the procedure was shorter in the TAVI group than in the SAVR group (1 [1–2] days versus 3 [2–4] days) and hospital stay after the procedure was also shorter in the TAVI group than in the SAVR group (8 [7–12] days versus 16 [13–23] days) (Table 2).
In-hospital death rate was numerically lower in the TAVI group than in the SAVR group (0.6% versus 2.2%). The rate of stroke was similar in the TAVI and SAVR groups (2.8% versus 2.1%). The rates of major bleeding and newly diagnosed AF were much lower in the TAVI group than in the SAVR group (20% versus 72%, and 4.6% versus 26%, respectively). In contrast, the rate of pacemaker implantation was higher in the TAVI group than in the SAVR group (8.1% versus 2.4%) (Table 2). The rate of pacemaker implantation in the TAVI group was higher with self-expandable valve than with balloon-expandable valve (13.1% versus 6.0%). The rate of acute kidney injury was numerically lower in the TAVI group than in the SAVR group (3.0% versus 5.5%).
Among patients without dialysis, in-hospital death rate was very low and comparable in the TAVI and SAVR groups (0.6% versus 0.8%) (Supplemental table 7). In contrast, among 105 patients with dialysis in the SAVR group, in-hospital death rate was high (8.6%), and the rates of disabling stroke and major vascular complications were much higher than those in patients without dialysis (Supplemental table 7 and 8). Among patients who underwent SAVR, in-hospital death rate of patients without concomitant procedure was numerically very low and lower than those with concomitant procedure (0.3% versus 4.3%) (Supplemental table 9). Among patients who underwent AVR for BAV, in-hospital death and disabling stroke rates were numerically higher in the TAVI group than in the SAVR group (2.9% versus 0.8%, and 5.9% versus 0.0%) (Supplemental table 10). With respect to patients stratified based on the STS-score risk stratum, the in-hospital mortality rate was remarkably high in SAVR group with high risk. In contrast, the in-hospital mortality rate in TAVI group was favorable even among patients deemed high risk. Moreover, even among low-risk patients, the in-hospital mortality rate was numerically lower in the TAVI group (Supplemental table 11). Among patients with CAD or any valvular disease, in-hospital death was numerically much higher in the SAVR group than in the TAVI group (4.4% versus 0.4%, 5.6% versus 0.4%) (Supplemental table 12, 13).
Echocardiographic data at discharge
Echocardiographic data at discharge was available in 564 patients (97.2%) in the SAVR group and in 1100 patients (97.1%) in the TAVI group. Vmax and mean PG were higher in the SAVR group than in the TAVI group and EOA was larger in the TAVI group than in the SAVR group. Left ventricular ejection fraction (LVEF) and stroke volume were greater in the TAVI group than in the SAVR group. Patient-prosthesis mismatch was more often seen in the SAVR group than in the TAVI group (moderate: 26% versus 9.0%, and severe: 4.8% versus 2.6%) (Table 3).
Discussion
The main findings of this study were the following: 1) Overall, among 3369 patients with severe AS enrolled from 21 centers, initial TAVI and initial SAVR strategies were chosen in 34.0% and 17.6% of patients, respectively; 2) Patients in the TAVI group were much older and had higher surgical risk scores than patients in the SAVR group, while patients with dialysis were almost exclusively treated with SAVR; 3) In-hospital death rate was numerically lower in the TAVI group than in the SAVR group. Among patients without dialysis, In-hospital death rate in patients was very low and comparable in the TAVI and SAVR groups (0.6% versus 0.8%), while in-hospital death rate in patients with dialysis was high after SAVR (8.6%); 4) In-hospital death rate of patients without concomitant procedure was numerically very low in the SAVR group (0.3%); 5) Among patients with BAV who underwent AVR, in-hospital death and stroke rates were numerically higher in the TAVI group than in the SAVR group (2.9% versus 0.8%, and 5.9% versus 0.0%); 6) Regarding the echocardiographic data at discharge, EOA, LVEF, and stroke volume were greater in the TAVI group than in the SAVR group, and the prevalence of patient-prosthesis mismatch was higher in the SAVR group than in the TAVI group.
The prevalence of initial AVR strategy in the present study (51.7%) was much higher than that in the CURRENT AS-1 registry (31.4%) conducted in the pre-TAVI era [10]. Moreover, the number of patients who underwent TAVI was much higher than the number of patients who underwent SAVR in this study. Suboptimal prevalence of SAVR had been one of an important issues in the management of patients with symptomatic severe AS [12]. Introduction of TAVI undoubtedly increased the prevalence of AVR particularly in patients with high or prohibitive surgical risk.
Currently, TAVI has been selected even in younger patients in the US after the low-risk trials [7]. In contrast, there was still a large difference in age between the TAVI and SAVR groups in the present Japanese study, and patients who underwent TAVI in this study were older than those in the US national registry [7]. Nevertheless, the in-hospital death rate after TAVI was numerically lower than that after SAVR. However, when considering the very low incidence of in-hospital mortality in patients without dialysis and patients without a concomitant procedure, the higher incidence of in-hospital mortality in the SAVR group was largely attributed to patients with dialysis or concomitant procedures.was very low and comparable to that after SAVR despite higher risk profiles of patients in the TAVI group than in the SAVR group. The in-hospital death rate after SAVR in the present study was comparable to that reported in the prior Japan Cardiovascular Surgery Database and in the real-world data in the US [13]. Especially, the in-hospital death rate in patients who underwent SAVR without concomitant procedure was quite low in this study.
The in-hospital death and stroke rates in the TAVI group in the present study were numerically lower than those reported in the prior Japanese TAVI registry conducted early after introduction of TAVI [14]. Moreover, the in-hospital death rate after TAVI in the present study was lower than those reported in European/US countries [7]. Considering the comparable in-hospital death rate in both the SAVR and TAVI groups, both strategies would be good interventional options for patients with severe AS. Nevertheless, the rates of new-onset atrial fibrillation and major bleeding were much higher in the SAVR group than in the TAVI group consistent with the prior studies [5, 6]. Regarding the echocardiographic data at discharge, the incidence of patient-prosthesis mismatch was lower in the TAVI group than in the SAVR group. The clinical impact of patient-prosthesis mismatch after TAVI has been still controversial [15]. However, several studies demonstrated the impact of moderate or severe patient-prosthesis mismatch after SAVR on worse long-term clinical outcomes [16]. Further follow-up would be warranted to evaluate very long-term clinical outcomes after SAVR and TAVI in terms of valve durability and the impact of patient-prosthesis mismatch. Nevertheless, given its less invasive nature and lower rates of procedural complications, TAVI might be the preferred treatment even in younger patients with severe AS who are currently treated with SAVR.
In contrast with the favorable in-hospital outcomes after SAVR in patients without dialysis, the in-hospital death rate in patients with dialysis was high after SAVR. The high rate of in-hospital death after SAVR in patients with dialysis in the present study was consistent with those in the previous studies [17]. In the present study, we could not evaluate the outcomes after TAVI in patients with dialysis, because TAVI for patients with dialysis was not approved in Japan during the enrollment period. The Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) TVT (Transcatheter Valve Therapies) registry revealed that patients with dialysis had higher risk of in-hospital death after TAVI than those without dialysis [18]. A large study would be warranted to evaluate clinical outcomes and valve durability in patients with dialysis who underwent TAVI and SAVR.
Regarding patients with BAV, in-hospital death rate and stroke were numerically lower in the SAVR group than in the TAVI group. Nevertheless, considering the large differences in age and patient characteristics between the TAVI and SAVR groups, both SAVR and TAVI would be reasonable options for selected patients with BAV [19]. Further study would be warranted to evaluate clinical outcomes for patients with BAV who underwent TAVI and SAVR.
Study limitations
There were several limitations in this study. First, among 21 participating centers, TAVI was not available in 11 centers, and SAVR was not available in 1 center. Therefore, the decision on proceeding to AVR and choice between TAVI and SAVR must have been affected by the availability of TAVI and SAVR in the individual centers. Second, we did not perform statistical testing for the comparison of clinical outcomes, because the selection of treatment must have been influcenced by factors other than the measured characteristics, and the low incidences of the clinical events did not allow the appropriate adjustment for the large differences in the baseline characteristics between the SAVR and TAVI groups. Third, TAVI for patients with dialysis was not approved in Japan during the study.
Conclusions
In this real-world data in Japan, TAVI compared with SAVR was chosen in much older patients with severe AS. In-hospital death rate was numerically lower in the TAVI group than in the SAVR group. Among patients without dialysis, In-hospital death rate in patients was very low and comparable in the two groups.
References
Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, Schmitz W, Kübler W. The effect of aortic valve replacement on survival. Circulation. 1982;66(5):1105–10. https://doi.org/10.1161/01.CIR.66.5.1105.
Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, PARTNER Trial Investigators, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607.
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, PARTNER Trial Investigators, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98.
Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–20.
Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–15.
Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–705.
Carroll JD, Mack MJ, Vemulapalli S, Herrmann HC, Gleason TG, Hanzel G, et al. STS-ACC TVT registry of Transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:2492–516.
Mori M, Gupta A, Wang Y, Vahl T, Nazif T, Kirtane AJ, et al. Trends in transcatheter and surgical aortic valve replacement among older adults in the United States. J Am Coll Cardiol. 2021;78:2161–72.
Kamon T, Kaneko H, Kiriyama H, Itoh H, Fujiu K, Kumazawa R, et al. Transcatheter aortic valve implantation and surgical aortic valve replacement for aortic stenosis in Japan - analysis of a nationwide inpatient database. Circ Rep. 2020;2(12):753–8.
Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, CURRENT AS Registry Investigators, et al. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2015;66:2827–38.
Takeji Y, Taniguchi T, Morimoto T, Shirai S, Kitai T, Tabata H, et al, (2020) CURRENT AS Registry-2 Investigators. Rationale, Design, and Baseline Characteristics of the CURRENT AS Registry-2. Circ J 86 (11) 1769–1776.
De Sciscio P, Brubert J, De Sciscio M, Serrani M, Stasiak J, Moggridge GD. Quantifying the shift toward transcatheter aortic valve replacement in low-risk patients. Circ Cardiovasc Qual Outcomes. 2017;10(6): e003287.
Thourani VH, Suri RM, Gunter RL, Sheng S, O’Brien SM, Ailawadi G, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg. 2015;99:55–61.
Takimoto S, Saito N, Minakata K, Shirai S, Isotani A, Arai Y, et al. Favorable clinical outcomes of transcatheter aortic valve implantation in Japanese patients - first report from the post-approval K-TAVI registry. Circ J. 2016;81:103–9.
Herrmann HC, Daneshvar SA, Fonarow GC, Stebbins A, Vemulapalli S, Desai ND, et al. Prosthesis-patient mismatch in patients undergoing transcatheter aortic valve replacement: From the STS/ACC TVT registry. J Am Coll Cardiol. 2018;72:2701–11.
Head SJ, Mokhles MM, Osnabrugge RLJ, Pibarot P, MacK MJ, Takkenberg JJM, et al. The impact of prosthesispatient mismatch on long-term survival after aortic valve replacement: A systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J. 2012;33:1518–29.
Yamauchi T, Yamamoto H, Miyata H, Kobayashi J, Masai T, Motomura N. Surgical aortic valve replacement for aortic stenosis in dialysis patients - analysis of japan cardiovascular surgery database. Circ J. 2020;84:1271–6.
Szerlip M, Zajarias A, Vemalapalli S, Brennan M, Dai D, Maniar H, et al. Transcatheter aortic valve replacement in patients with end-stage renal disease. J Am Coll Cardiol. 2019;73:2806–15.
Vincent F, Ternacle J, Denimal T, et al. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Circulation. 2021;143(10):1043–61.
Funding
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Dr. Shiomi reports personal fees from Abbott Vascular, Boston Scientific, and Daiichi Sankyo. Dr. Morimoto reports lecturer's fees from Bristol-Myers Squibb, Daiichi Sankyo, Japan Lifeline, Kowa, Kyocera, Novartis, and Toray; manuscript fees from Bristol-Myers Squibb and Kowa; advisory board for Sanofi. Dr. Kimura reports advisory board for Abbott Vascular; grants from Edwards Lifescience, Daiichi Sankyo, Takeda Pharmaceutical, Bayer, Otsuka Parmaceutical, Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Sumitomo Dainippon Pharma, Kowa, Abiomed, Japan Academic Research Forum, NIPRO, W. L. Gore & Associates G.K., RPM Co.,Ltd., CSL Behring, Pfizer R&D Japan G.K., EP-CRSU Co., Ltd; modest honoraria from MSD, Eisai, Edwards Lifescience, Ono Pharmaceutical, Tsumura, Medical Review, Kowa, Sanofi, Pharmaceuticals and Medical Devices Agency, Bristol-Myers Squibb, Boston Scientific, Lifescience, Toray, Astellas Amgen Biopharma, Astellas, AstraZeneca, OrbusNeich, MSD Life Science Foundation, Public Health Research Foundation, Chugai Pharmaceutical, Japan Society for the Promotion of Science, Interscience, Philips, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, Terumo, Novartis Pharma, HeartFlow Japan G.K., CROSSCO Co.
IRB information
The present study was approved by Kyoto University Graduate School and Faculty of Medicine Ethics Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takeji, Y., Taniguchi, T., Morimoto, T. et al. In-hospital outcomes after SAVR or TAVI in patients with severe aortic stenosis. Cardiovasc Interv and Ther 39, 65–73 (2024). https://doi.org/10.1007/s12928-023-00942-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-023-00942-x