Abstract
The aim of this study is to evaluate the long-term safety and efficacy of the 2.25 mm bioresorbable-polymer sirolimus-eluting Ultimaster stent in a Japanese patient population. Treatment of coronary artery disease in very small vessels is associated with an increased risk for cardiac events. The CENTURY JSV study is a prospective, multicenter, single-arm study. Seventy patients with stable and unstable coronary artery disease with a coronary lesion eligible for implantation with a 2.25 mm stent were enrolled in this study. Patients underwent clinical follow-up through 5-year after the PCI procedure. The mean age was 70.4 ± 9.2 years. The prevalence of diabetes mellitus was 37.1%, all not insulin dependent. The incidence of major adverse cardiac events, defined as cardiac death, target vessel myocardial infarction (MI), and clinically driven target lesion revascularization (CD-TLR) at 5 years was 5.7%. A non-Q wave MI was noted in 1.4% and 4.3% underwent a CD-TLR. There was no stent thrombosis during the entire follow-up period. No cardiac events were reported between 2 and 5 years. This is the first study to demonstrate safety and effectiveness for 5 years after treatment of very small coronary disease with 2.25 mm-diameter DES.
Clinical trial registration: UMIN000012928
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of small vessel treatment among patients undergoing a percutaneous coronary intervention (PCI) has been reported to be around 35–50% of the cases [1,2,3,4]. For the interventional cardiologist, small vessel PCI constitute a technical challenge as small vessels are more distally located in the coronary system with frequently a diffuse distribution of the atherosclerotic narrowing’s rather than a discrete lesion [5, 6]. Moreover, small vessel disease is associated with an increased risk for a cardiac event, including restenosis and stent thrombosis [2, 4]. The introduction of drug eluting stents (DES) has improved the outcomes versus bare metal stents due to the inhibition of in-stent neointimal proliferation resulting in a lower in-stent late lumen loss and so maintaining stent patency, even in small diameter stents which are more prone to restenosis [7, 8]. These promising outcomes and technical developments have started the development of stents suitable for the treatment of vessels < 2.5 mm. The 2.25 mm-diameter Ultimaster stent is an extension of the regular sizes that has been evaluated in a comprehensive clinical program [9, 10].
The current CENTURY JSV study was initiated to evaluate the safety and efficacy of the 2.25 mm-diameter Ultimaster stent and the 9-month angiographic and 2-year clinical outcomes have been reported earlier [11]. This update report has the final 5-year outcomes.
Methods
Study design and patient population
The design of the CENTURY JSV study has been published earlier [11]. Briefly, it was a prospective, multicenter, single-arm study that enrolled patients with a minimum age of 20 years with asymptomatic myocardial ischemia, stable or unstable angina pectoris with a lesion suitable for implantation of a single 2.25 mm-diameter Ultimaster stent. Angiographic follow-up was performed at 9-month follow-up. Clinical follow-up was performed at 1 and 9 months, 1 year and annually up to 5 years (Fig. 1).
The target lesion had to be covered with one stent (maximum length 38 mm). Up to two non-target lesions could be treated during the index procedure provided that non-study stent implantation was uncomplicated before continuing the procedure with pre-dilatation of the target lesion. Patients with an acute myocardial infarction (MI) within 48 h before the procedure, renal failure requiring dialysis, left-ventricular ejection fraction < 25%, life expectancy < 1 year, or patients requiring a staged procedure were excluded. Anatomical exclusion criteria included bifurcation lesions requiring stenting of both main and side branch, ostial lesions, arterial and venous bypass grafts, left main lesions, in-stent restenosis, and lesion requiring preparation other than balloon pre-dilation. The study was performed according to the Declaration of Helsinki and Good Clinical Practice, and all patients provided written informed consent. The Institutional Review Board of each participating site approved the study. The UMIN-CTR clinical trial registration number is UMIN000012928.
Study device
The Ultimaster stent (Terumo Corporation, Tokyo, Japan) is a thin-strut (80 μm) cobalt–chromium sirolimus-eluting stent with an open-cell design [9]. Sirolimus is embedded in a biodegradable polymer coating (poly-D, L-lactic acid polycaprolactone) that is fully metabolized through dl-lactide and caprolactone into carbon dioxide and water in 3–4 months. The gradient coating reduces polymer cracking and delamination at the stent hinges. The coating is applied on the abluminal side of the struts only, and a bare metal stent remains after resorption of the coating in 3–4 months. For the current study, stent lengths of 12, 15, 18, 24, 28, 33, and 38 mm were available with a diameter of 2.25 mm.
Procedural and post-interventional practices
Apart from mandatory lesion pre-dilatation, PCI was performed in accordance with the standard procedure of each hospital. Lesion preparation with other devices than pre-dilatation balloons was an exclusion criterion. The aim was to cover the lesion with a single stent. In case of a bail-out situation, implantation of additional stents was allowed. Post-dilatation was at the discretion of each operator. Antiplatelet therapy with aspirin and a P2Y12 inhibitor was started before the index procedure and maintained for a minimum of 9 months.
Follow-up, study endpoints, and definitions
The primary endpoint was the incidence of major adverse cardiac events (MACE) at 9 months, defined as cardiac death, target vessel MI, and clinically driven target lesion revascularization (TLR). Cardiac death was defined as any death due to proximate cardiac cause (e.g., MI, low-output failure, fatal arrhythmia), unwitnessed death and death of unknown cause, and all procedure-related deaths, including those related to concomitant treatment. MI was defined either as the development of pathological Q-waves in at least two contiguous leads with or without elevated cardiac enzymes or, in the absence of pathological Q-waves, as an elevation in creatinine kinase levels to greater than twice the upper limit of normal in the presence of an elevated level of CK-MB fraction or troponin. Cardiac biomarkers were obtained before the procedure and after the procedure in intervals of 6 h up to 24 h to detect myocardial injury. TLR was defined as repeat percutaneous intervention of the stented lesion including 5 mm proximal and distal from the edge of the stent, or bypass surgery of the target vessel that was performed for a clinical indication and was due to restenosis or closure of the target lesion. A revascularization was considered clinically indicated if prompted by a positive functional study, or ischemic ECG changes at rest in a distribution consistent with the target vessel, or ischemic symptoms with an in-lesion diameter stenosis ≥ 50% by QCA or if lesion diameter stenosis was more than 70% at follow-up, even in the absence of clinical symptoms. Target lesion failure (TLF) was defined as cardiac death that cannot be clearly attributed to a vessel other than the target vessel, target vessel MI, and clinically driven target lesion revascularization. Target vessel failure (TVF) was defined as cardiac death that cannot be clearly attributed to a vessel other than the target vessel, target vessel MI, and clinically driven target vessel revascularization. Stent thrombosis and bleedings were defined by the Academic Research Consortium and Bleeding Academic Research Consortium (BARC) definitions, respectively [12, 13]. Stent thrombosis was picked up definite, probable and possible stent thrombosis. Bleeding events were picked up only BARC 3 and 5 bleeding. All events were adjudicated by a Clinical Events Committee.
Statistical analysis
Continuous variables are reported as means along with the standard deviation (SD). Categorical variables are reported as frequencies and percentages. The cumulative event free rates as a function of time were calculated by the Kaplan-Meier method. All the analyses were carried out using SAS version 9.4 (SAS Institute, Japan Ltd.).
Results
Baseline and procedural characteristics
The study enrolled 70 patients between April 16 and December 25, 2014 by seven sites in Japan. The enrolled patients represented a typical population requiring a PCI with a mean age of 70.4 ± 9.2 years and a predominantly male gender (77.1%). The prevalence of diabetes mellitus was 37.1%, but none of the patients required insulin treatment. Regarding other classic cardiovascular risk factors, hypertension and dyslipidemia were present in 87.1% of the patients. Previous smoking was reported by 42.9% of the patients; 11.4% of the patients was a current smoker. A previous PCI was performed in 52.9%. Table 1 shows an overview of the co-morbidities. The location of the target lesions was the left circumflex in 42.9% and the left anterior descendants in 32.9% of the patients. A bifurcation was involved in 21.4%. The mean lesion length was 14.6 ± 7.6 mm, and the mean reference vessel diameter was 2.0 ± 0.3 mm. In total, 72 Ultimaster 2.25 mm stents were implanted; two (2.9%) patients required an extra study stent because of a bail-out situation. The mean length of the implanted Ultimaster stents was 21.4 ± 8.2 mm. Post-dilatation was performed in 75.7% resulting in a mean post procedural in-stent diameter stenosis of 11.7 ± 8.7%. Summary of lesion and procedural characteristics are shown in Table 2.
Clinical outcomes
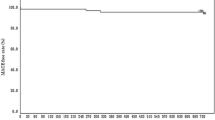
All patients were followed up to 5 years except 4 patients who died due to non-cardiac causes. An MACE was observed in 4 patients (5.7%) throughout 5 years. No cardiac death was reported. One patient experienced non-Q MI and three patients underwent a clinically driven TLR. All these events occurred before the 2-year follow-up. None of the patients reported an MACE between 2 and 5 years. Furthermore, there were no instances of stent thrombosis (as defined by the Academic Research Consortium) through 5 years. TLF and TVF were observed in 4 patients (5.7%) and 6 patients (8.6%) at 5 years, respectively. Bleeding evens were observed in 3 patients (4.3%) at 5 years after the procedure (Table 3). Kaplan–Meier curves for each clinical event are drawn in Fig. 2.
Discussion
To the best of our knowledge, this is the first study to demonstrate the 5-year safety and efficacy of very small vessel PCI with 2.25-mm DES. In detail, there were no cardiac death, one (1.4%) non-Q wave MI, and three (4.3%) clinically driven TLRs. All events occurred in the first 2 years; no cardiac events were reported between 2 and 5 years. Notably, there were no stent thrombosis during the entire follow-up period.
Coronary vessel diameter has a continuous inverse relationship with procedural complexity and the incidence of cardiac events [4, 14, 15]. Correspondingly, the definitions of what constitute a small vessel is arbitrary, varies between studies and has evolved over time [6]. Initially, in the early days of bare metal stents, vessels ≤ 2.9 mm in lumen diameter were considered small [16]. Over time, with the introduction of DES, the upper threshold has been lowered to 2.75 or 2.5 mm, or even lower depending on the trial [4, 17]. Based on cardiac event rates with novel DES, a vessel diameter of 2.5 mm has been suggested to identify small target vessels [2]. The advent of DES technology with small strut designs as well as the availability of low-profile delivery balloons allowed the design of 2.0- and 2.25-mm stent iterations to treat very small vessels and introduced corresponding terminology to distinguish these vessels from the ‘regular’ small vessels [8, 11, 18].
The therapeutic options for (very) small vessels are similar as for all coronary artery disease and include medical therapy and revascularization by PCI or coronary artery bypass grafting (CABG). Guidelines recommend CABG for patients with multivessel disease and higher Syntax scores [19, 20]. However, small vessel disease, often characterized by diffuse atherosclerosis over a longer segment of the distal vessel, constitutes a technical challenge to anastomose the graft with sufficient run-off with consequently an increased risk for an incomplete revascularization and cardiac events [21]. Similarly, a distal lesion can be difficult to reach for a PCI, and diffuse disease over longer segments in multiple vessels reduces the chance for a successful procedure [3]. In addition, small vessel disease remains an independent predictor of MACE after PCI [22]. Careful decision-making by the Heart Team, weighing the pros and cons of the therapeutic options including the patient’s preference, is the cornerstone of contemporary patient management for patients with challenging anatomies such as small vessel disease.
The result of the current study demonstrates that PCI is a suitable treatment option for selected patients with very small vessel disease. All stents were successfully implanted, and the peri-procedural cardiac event rate (1.4%) was low. During the 5-year follow-up period, no MACE events occurred after 2 years resulting in a final low MACE rate of 5.7%. Ultimaster stents are designed, such that their polymers (poly DL-lactic acid) are absorbed over the course of 3–4 months. After that, the polymer was completely dissolved, and the less inflammatory bare metal surface came into direct contact with the vascular wall, accommodating relatively healthy and homogeneous neointimal tissue growth for the remainder. In addition, Wilson et al. [23] demonstrated that the use of a bioresorbable-polymer coating as a method for drug elution results in lower long-term inflammation compared to durable polymer DES. Furthermore, Itoh et al. [24] showed that qualitatively and quantitatively consistent neointimal stent coverage was achieved by the 12-month time point by optical frequency-domain imaging. After the polymer was completely dissolved, and the less inflammatory bare metal surface came into direct contact with the vascular wall, accommodating relatively healthy and homogeneous neointimal tissue growth. These reactions might affect the absence of MACE and TLR from 2 years on.
A clinically driven TLR rate of 4.3% is impeccable, as even small amounts of intimal hyperplasia can cause functional or anatomical restenosis within a narrow stent lumen. The in-stent late lumen loss of 0.22 ± 0.31 mm and the in-stent binary restenosis rate of 4.3% at 9 months earlier reported for this study are re-assuring in that perspective and provide an angiographic substantiation for the clinical outcomes [11].
Comparison with the study results for small vessels conducted in the past
Kandzari et al. [25] reported that cardiac death or MI in the small vessel (≤ 2.25 mm) subgroup of the PROMUS Element Plus US Post-Approval Study was 13% and TLF was 16%. Pilgrim et al. [26] reported in a BIOSCIENCE randomized trial that the TLFs of BP-SES and DP-SES for small vessels (defined as stent diameter in any lesion ≤ 3 mm) were 20.3% and 18.4% (P = 0.241), respectively. Lefèvre et al. [27] reported in BIO FLOW-II that the TLFs of O-SES and X-EES for small vessels (≤ 2.75 mm) were 11.1% and 15.5% (P = 0.303), respectively. Kelly et al. [28] reported that the TLF of Pt-Cr EES for small vessels (diameter < 2.5 mm) was 7.0% in the PLATINUM Trial. Event rates were above 10% in most trials. These results show that even when DES are used, treatment of very small vessels can still be challenging depending on patient and lesion-specific characteristics.
Similar to our study, Saito et al. [29] reported 4-year results of the RESOLUTE Small Vessel Study that evaluated Resolute in Japanese patients (RESOLUTE Japan SV study). There were some differences of patient and lesion characteristics between CENTURY JSV study and RESOLUTE Japan SV study. Rate of IDDM was significantly higher in RESOLUTE Japan SV study (10.8 vs. 0%); in the other hand, ratio of B2 and C in lesion classification was higher in CENTURY JSV study (68.6 vs. 45.1%). There is no difference in other characteristics. The MACE rate at 2 years of RESOLUTE Japan SV was 5.4% but increased over time to 12.3% at 4 years. Similarly, the TLF of the small vessel group of PERSEUS SV Trial and BIOFLOW-II also increased over time. Konigstein et al. [15] demonstrated that reference vessel diameter was the only lesion-related predictor of long-term TLF from individual patient data pooled analysis from 6 randomized controlled trials. The reference vessel diameter of the treated vessels in our study was very small (1.95 ± 0.28 mm). Despite implanting in such small vessels, it is a remarkable result that the 5-year MACE was extremely low at 5.7%; additionally, there is no subsequent occurrence from 2 year follow-up.
Comparison between drug-coated balloon (DCB) and DES in small vessels
Regarding strategy for native small vessel disease, there is conflicting evidence about the effects of DCB compared with DES in patients with native small vessel disease. The PICCOLETO study which randomized between first-generation DCB and DES reported a trend toward higher major adverse cardiovascular events rate at 9 months in the DCB group [30]. On the other hand, new-generation DCB studies showed that DCB use was associated with comparable risk of TLR and MACE in the BELLO study [31], RESTORE study [32], and BASKET-SMALL 2 study [33]. The recent PICCOLETO II trial showed that new-generation DCB was found superior to DES in terms of late lumen loss by angiographic follow-up [34]. However, the limitations of these trial were not large number of patients and not powered for clinical endpoints. An SCAAR report, including the largest real-world population with small vessel disease, showed that DCB was associated with approximately double the risk for restenosis at long-term follow-up compared with new-generation DES even after propensity score matching for baseline characteristics [35]. DCB use should be therefore limited to selected cases for which stent implantation is not desirable.
Strengths and limitations
The study was designed as a single-arm study and comparison of outcomes with other treatment options is limited by inherent design differences between studies. A single stent designed for very small vessels was evaluated and the results can therefore not be extrapolated to other stent models. The angiographic evaluation at 9 months allows to correlate these findings with the clinical outcomes. The 5-year follow-up is sufficiently long to assess the long-term outcomes. The number of patients is low and has been selected according to protocol criteria limiting the generalization to a larger population of patients with small vessel disease.
Conclusions
The final 5-year results of the CENTURY JVS study establish the long-term safety and effectiveness of the 2.25 mm Ultimaster bioresorbable-polymer sirolimus-eluting stent for the treatment of very small coronary disease in Japanese patients.
Abbreviations
- DES:
-
Drug-eluting stent
- MACE:
-
Major adverse cardiac events
- MI:
-
Myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- TLR:
-
Target lesion revascularization
- TLF:
-
Target lesion failure
- TVF:
-
Target vessel failure
References
Wöhrle J, Markovic S, Rottbauer W, Muramatsu T, Saito S, Wijns W, et al. Bioresorbable polymer sirolimus-eluting coronary stent compared with permanent polymer everolimus-eluting coronary stent implantation for treatment of small vessel coronary artery disease: CENTURY II trial. EuroIntervention. 2016;12(2):e167–74.
van der Heijden LC, Kok MM, Danse PW, Schramm AR, Hartmann M, von Birgelen C, et al. Small-vessel treatment with contemporary newer-generation drug-eluting coronary stents in all-comers: Insights from 2-year DUTCH PEERS (TWENTE II) randomized trial. Am Heart J. 2016;176:28–35.
Schunkert H, Harrell L, Palacios IF. Implications of small reference vessel diameter in patients undergoing percutaneous coronary revascularization. J Am Coll Cardiol. 1999;34:40–8.
Danenberg HD, Konigstein M, Golomb M, Kandzari DE, Smits PC, Stone GW, et al. Incidence and predictors of target lesion failure in patients with lesions in small vessels undergoing PCI with contemporary drug-eluting stents: insights from the BIONICS Study. Cardiovasc Revasc Med. 2021;25:1–8.
Ertan C, Ozeke O, Gul M, Aras D, Topaloglu S, Ozin B, et al. Association of prediabetes with diffuse coronary narrowing and small-vessel disease. J Cardiol. 2014;63:29–34.
Wybraniec MT, Bańka P, Bochenek T, Roleder T, Mizia-Stec K. Small vessel coronary artery disease: how small can we go with myocardial revascularization? Cardiol J. 2021;28:767–78.
Puymirat E, Mangiacapra F, Peace A, Sharif F, Conte M, Barbato E, et al. Long-term clinical outcome in patients with small vessel disease treated with drug-eluting versus bare-metal stenting. Am Heart J. 2011;162:907–13.
Biondi-Zoccai G, Moretti C, Abbate A, Sheiban I. Percutaneous coronary intervention for small vessel coronary artery disease. Cardiovasc Revasc Med. 2010;11:189–98.
Saito S, Valdes-Chavarri M, Richardt G, Moreno R, Iniguez Romo A, Wijns W, et al. CENTURY II investigators. a randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (Clinical evaluation of new Terumo drug-eluting coronary stent system in the treatment of patients with coronary artery disease) trial. Eur Heart J. 2014;35:2021–31.
Mohamed MO, Polad J, Hildick-Smith D, Bizeau O, Baisebenov RK, Mamas MA, et al. Impact of coronary lesion complexity in percutaneous coronary intervention: one-year outcomes from the large, multicentre e-ultimaster registry. EuroIntervention. 2020;16:603–12.
Saito S, Ando K, Ito Y, Tobaru T, Yajima J, Kimura T, et al. Two-year results after coronary stenting of small vessels in Japanese population using 2.25-mm diameter sirolimus-eluting stent with bioresorbable polymer: primary and long-term outcomes of CENTURY JSV study. Cardiovasc Interv Ther. 2019;34:25–33.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Serruys PW, et al. Academic research consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, White H, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–47.
Wykrzykowska JJ, Serruys PW, Onuma Y, de Vries T, van Es GA, Windecker S, et al. Impact of vessel size on angiographic and clinical outcomes of revascularization with biolimus-eluting stent with biodegradable polymer and sirolimus-eluting stent with durable polymer the LEADERS trial substudy. JACC Cardiovasc Interv. 2009;2(9):861–70.
Konigstein M, Madhavan MV, Ori B-Y, Rahim HM, Iva S, Gkargkoulas F, et al. Incidence and predictors of target lesion failure in patients undergoing contemporary DES implantation—Individual patient data pooled analysis from 6 randomized controlled trials. Am Heart J. 2019;213:105–11.
Doucet S, Schalij MJ, Vrolix MC, Hilton D, Chenu P, Lespérance J, et al. Stent in small arteries (SISA) trial investigators. Stent placement to prevent restenosis after angioplasty in small coronary arteries. Circulation. 2001;104:2029–33.
Dan K, Garcia-Garcia HM, Kolm P, Windecker S, Saito S, Waksman R, et al. Comparison of ultrathin, bioresorbable-polymer sirolimus-eluting stents and thin, durable-polymer everolimus-eluting stents in calcified or small vessel lesions. Circ Cardiovasc Interv. 2020;13: e009189.
Jen HL, Wang YC, Tsao TP, Yin WH. Percutaneous coronary intervention for very small vessels with the use of a newer-generation 2.0 mm drug-eluting stent. J Invasive Cardiol. 2021;33:E565–74.
Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Smith PK, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American college of cardiology appropriate use criteria task force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, society for cardiovascular angiography and interventions, society of cardiovascular computed tomography, and society of thoracic surgeons. J Am Coll Cardiol. 2017;69:2212–41.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Zembala MO, et al. ESC scientific document group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165.
O’Connor NJ, Morton JR, Birkmeyer JD, Olmstead EM, O’Connor GT. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Northern New England cardiovascular disease study group. Circulation. 1996;93:652–5.
Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Kastrati A, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100(2):153–9.
Wilson GJ, McGregor J, Conditt G, Shibuya M, Sushkova N, et al. Impact of bioresorbable versus permanent polymer on longterm vessel wall inflammation and healing: a comparative drug-eluting stent experimental study. EuroIntervention. 2018;13:1670–9.
Itoh Y, Otake H, Kimura T, Tsukiyama Y, Kikuchi T, et al. A serial optical frequency-domain imaging study of early and late vascular responses to bioresorbable-polymer sirolimus-eluting stents for the treatment of acute myocardial infarction and stable coronary artery disease patients: results of the MECHANISM-ULTIMASTER study. Cardiovasc Interv Ther. 2022;37(2):281–92.
Kandzari DE, Amjadi N, Caputo C, Rowe SK, Chen H, Williams J, et al. Final 5-year results in unselected patients implanted with a thin-strut, platinum-chromium, everolimus-eluting stent (from the PROMUS element plus US post-approval study). Am J Cardiol. 2019;123:1765–71.
Pilgrim T, Piccolo R, Heg D, Roffi M, Tuller D, Muller O, et al. Ultrathin-strut, biodegradable-polymer, sirolimus-eluting stents versus thin-strut, durable-polymer, everolimus-eluting stents for percutaneous coronary revascularisation: 5-year outcomes of the BIOSCIENCE randomised trial. Lancet. 2018;392:737–46.
Lefèvre T, Haude M, Neumann FJ, Stangl K, Skurk C, Slagboom T, et al. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent 5-Year outcomes of the randomized BIOFLOW-II trial. JACC: Cardiovasc Interv. 2018;11(10):995–1002.
Kelly CR, Teirstein PS, Meredith IT, Farah B, Dubois CL, Feldman RL, et al. Long-term safety and efficacy of platinum chromium everolimus-eluting stents in coronary artery disease 5-year results from the PLATINUM trial. JACC: Cardiovasc Interv. 2017;10(23):2392–400.
Saito S, Maehara A, Vlachojannis GJ, Parise H, Mehran R. Clinical and angiographic evaluation of the resolute zotarolimus-eluting coronary stent in japanese patients – long-term outcome in the RESOLUTE Japan and RESOLUTE Japan small vessel study. Circ J. 2015;79:96–103.
Cortese B, Micheli A, Picchi A, Coppolaro A, Bandinelli L, et al. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO Study. Heart. 2010;96(16):1291–6.
ILatib A, Colombo A, Castriota F, Micari A, Coremonesi A, et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxeleluting stent in small coronary vessels: the bello (balloon elution and late loss optimization) study. J Am Coll Cardiol. 2012;60(24):2473–80.
Tang Y, Qiao S, Su X, Chen Y, Jin Z, et al. Drug-coated balloon versus drug-eluting stent for small-vessel disease: the RESTORE SVD China randomized trial. JACC Cardiovasc Interv. 2018;11(23):2381–92.
Jeger RV, Farah A, Ohlow MA, et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet. 2018;392(10150):849–56.
Cortese B, Palma GD, Guimaraes MG, Piraino D, Orrego PS, et al. Drug-coated balloon versus drug-eluting stent for small coronary vessel disease: PICCOLETO II randomized clinical trial. J Am Coll Cardiol Intv. 2020;13:2840–9.
Silverio A, Buccheri S, Venetsanos AJ, Lagerqvist B, et al. Percutaneous treatment and outcomes of small coronary vessels: a SCAAR report. J Am Coll Cardiol Intv. 2020;13:793–804.
Acknowledgements
None.
Funding
This work was funded by Terumo Corporation, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors wish to disclose the following conflicts of interest: SS, KA, KK, YI, TT, JY, and TK received grants from Terumo Corporation during the period that this study was conducted. KA received consultant fees from Terumo Corporation. JY and YI received honorarium from Terumo Corporation. TK received a research grant from Terumo Corporation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shishido, K., Ando, K., Ito, Y. et al. Five-year clinical outcomes of a 2.25 mm sirolimus-eluting stent in Japanese patients with very small coronary artery disease: final results of the CENTURY JSV study. Cardiovasc Interv and Ther 38, 194–201 (2023). https://doi.org/10.1007/s12928-022-00890-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-022-00890-y