Abstract
Spatial mark-recapture abundance estimates obtained from fecal genotyping are becoming an essential component of conservation of carnivores. The bobcat (Lynx rufus) is a widespread carnivore in California, USA, that until recently lacked robust demographic data. To facilitate a statewide abundance study, we created a single nucleotide polymorphism (SNP) genotyping panel for individual identification. For SNP discovery, we performed restriction site-associated DNA sequencing (RADseq) on 78 samples collected throughout California and subsequently designed a panel of 96 SNPs for sequencing on a microfluidic platform. This panel includes loci to identify sex and differentiate bobcats from other common carnivores. The panel reliably differentiates individuals when using DNA extracted from feces, with 89% of samples amplifying at > 90% of SNPs. Importantly, we found autosomal SNPs were monomorphic in the closely related Canada lynx (L. canadensis) suggesting the panel would still be effective for bobcat study in areas of sympatry. Fecal genotyping provides a cost-effective, noninvasive method for population monitoring and detecting individual movement. Our panel generates standardized genotypes that can be analyzed across laboratories and used for continued bobcat monitoring in California and other western states.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Conservation and management strategies for carnivores are contingent upon accurate demographic data. Fecal genotyping presents a cost-effective and noninvasive means of obtaining mark-recapture data that is often preferable for monitoring populations of elusive carnivores (Waits and Paetkau 2005). In California, the bobcat (Lynx rufus), a widespread carnivore, is the subject of recent conservation focus due to a lack of robust demographic data. The accurate estimation of bobcat populations is crucial for understanding their ecology and the effects of fragmented landscapes, particularly in the face of urban expansion and disease (Serieys et al. 2015; Kozakiewicz et al. 2019). To aid population estimates, we developed a single nucleotide polymorphism (SNP) genotyping panel designed to identify individual bobcats from noninvasively collected fecal samples.
Empirical studies have raised concerns about the accuracy of microsatellites when applied to fecal genotyping (Creel et al. 2003; Piggott 2004). Although SNPs and microsatellites can both provide high-quality genotypes from fecal samples with adequate quality control measures in place, SNPs tend to attain higher genotyping success rates than microsatellites, attributed to the shorter size of the target sequence (von Thaden et al. 2017; Ekblom et al. 2021). SNP datasets that have been vetted for use with degraded samples provided highly accurate genotypes (Schultz et al. 2018), in some cases outperforming microsatellites when directly compared (von Thaden et al. 2020; Ekblom et al. 2021). In contrast to microsatellites, SNPs have the added benefit of not requiring allele calling standardization across laboratories. Our SNP panel was designed using samples spanning California’s diverse ecoregions to maximize resolution throughout the state (Table S1). We included sex-linked SNPs and mitochondrial (mtDNA) loci differentiating bobcat from sympatric carnivores. The resulting panel of 96 highly reliable SNPs, optimized for a microfluidic platform, represents an effective, multipurpose genetic tool capable of distinguishing individual, sex, and species, even when applied to low-quality fecal DNA.
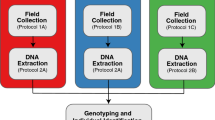
For SNP discovery, we extracted DNA from 78 bobcat tissues (Table S1) using the Qiagen Genomic-tip extraction kit (Qiagen Inc.,Valencia, CA). We used a genotyping-by-sequencing (GBS) approach for restriction site-associated DNA sequencing (RADseq) based on methods outlined in Elshire et al. (2011) using PstI high-fidelity restriction enzyme. Libraries were sent to the University of California, Davis Genome Center for Illumina sequencing on a NovaSeq 6000 platform which produced 150 bp paired-end reads. We demultiplexed reads and trimmed adapters using GBSX v.1.3 (Herten et al. 2015). We aligned reads to the Lynx canadensis reference genome (GCF_007474595.2_mLynCan4.pri.v2; GenBank Accession Nos. CM017329.2-CM017347.2., CM023961.1) using BWA-MEM and default parameters (Li 2013). SNP calling and filtering steps were performed in stacks (v.2.53; Catchen et al. 2011) using the ref_map.pl pipeline with the following filters in the “populations” module: minor allele frequency 0.3, observed heterozygosity ≤ 0.6, genotypes present in ≥ 90% individuals, and we merged sites represented in overlapping loci.
We then selected 190 candidate autosomal bobcat SNPs that were evenly spaced across the genome, that had no indels, and had ≤ 1 SNP present in 75 bp of adjacent sequence in either direction (3’, 5’) to minimize the risk of mutations in primer binding sites. Further, we designed five taxon- or species-diagnostic mtDNA markers to discriminate between bobcats and other common carnivores. We aligned mtDNA sequences obtained from GenBank (Table S2) using mega v.10.0.5 and selected loci within the 12S or 16S ribosomal RNA genes that possessed a variable site flanked by highly conserved regions across carnivore taxa, with target amplicon lengths of < 130 bp.
We used the D3 Assay Design Tool (https://d3.standardbio.com) to create Standard BioTools (formerly, Fluidigm; South San Francisco, CA) compatible oligos for all 195 markers. We validated the panel using a separate set of 67 bobcat tissue samples (Table S1) and carnivore reference samples (Table S3). We followed manufacturer protocols for the integrated fluidic circuit (IFC) 96.96 Dynamic Array. All IFCs were run on the Standard BioTools Juno thermocycler and the Biomark HD system and subsequently analyzed using their SNP Genotyping Analysis Software v.4.5.1.
From the 190 candidate autosomal loci, we identified 163 SNPs that amplified well with the standard chemistry (Table S4 and S5). We then selected SNPs with optimal clustering and high amplification rates for inclusion in a single 96 panel IFC. Our final panel included 91 autosomal SNPs, two sex markers (2FelidSRYSNP-GT and mlbcZfy-680; Buchalski et al. 2022), and three mtDNA markers, capable of differentiating felids from other carnivores (FelVCan_2496_16SriboRNA), bobcat from mountain lion (mtdna_658; Buchalski et al. 2022), and other carnivores from coyote (Canis latrans; CoyVAll_2427_16SriboRNA), a common non-target species whose feces can be misidentified as bobcat during surveys (Tables S3, S4, S5). We also validated an additional felid diagnostic mtDNA marker and two red fox (Vulpes vulpes) diagnostic mtDNA markers which could be incorporated for studies with high red fox abundance.
To characterize the level of polymorphism of the panel, we used the combined SNP discovery and validation datasets (n = 145; Table S1; genotype file provided in Genepop format as Supplementary File 2) and excluded poor-quality genotypes (> 20% missing data). We used R v.4.3.1 (R Core Team 2023) and the adegenet package (Jombart and Ahmed 2011) to calculate expected heterozygosity and minor allele frequency. We then used popgenutils package to estimate the probability that two individuals (PID) or two siblings (PIDsibs) may have the same genotype (Tourvas 2021).
Our panel yielded a high (99%) amplification rate with 66 of 67 tissue samples successfully genotyped above the 20% missing data cutoff. The autosomal SNPs had high resolving power as reflected in the decreasing trend in PID and PIDsibs across loci, with an overall PID of 9.2 × 10–38 for the combined panel (Fig. 1, Table S6). Heterozygosity estimates ranged from 0.38 to 0.5 among loci, with minor allele frequencies between 0.25 and 0.5 (Table S6). The autosomal loci showed minimal cross-amplification in canids, raccoon (Procyon lotor), American badger (Taxidea taxus), and black bear (Ursus americanus), and monomorphic genotypes for non-target felids (Canada lynx, L. canadensis; mountain lion, Puma concolor; domestic cat, Felis catus; genotypes provided in Supplementary File 2). The mtDNA markers (Table S3) were accurate in differentiating felid species (102/102) from other tested carnivores, and bobcats from both red fox (13/13) and coyote (15/15). For locus RedVCoyGray_227_12SriboRNA we found a haplotype unique to red foxes suggesting this marker is species-diagnostic. However, the coyote marker shared haplotypes with two carnivores, American badger (14/14; T. taxus) and kit fox (1/10; V. macrotis).
Number of SNP loci in a genotype and resulting decrease in probability of identity (PID) and probability of sibling identity (PIDsibs) for 145 bobcats (Lynx rufus) sampled throughout California. Of 91 autosomal loci, only 1 to 14 are shown for clarity as PID approaches zero. Calculations of PID generated from 1000 permutations for random combinations of loci (pid_permute function in R package POPGENUTILS; Tourvas 2021). Both PID and PIDsibs calculations for individual loci and combined statistics for full panel provided in Table S6
We also tested the panel with 88 fecal samples extracted using the QIAamp Fast DNA Stool Mini Kit (Qiagen Inc.). We modified the 96.96 Dynamic Array protocol with PCR pre-amplification optimizations for low quality samples (von Thaden et al. 2020). Of the 88 samples, 83 were determined by mtDNA markers to be bobcat. Of those, 74 (89.2%) successfully genotyped (90–100% of SNPs called) and nine failed (< 40% SNPs called).
Our SNP genotyping panel represents an advancement in the demographic monitoring of bobcats. Simultaneous individual identification, sex determination, and differentiation from non-target carnivores enhances the efficacy and accuracy of abundance estimates based on fecal genotyping and allows for tracking bobcat movement over time periods that complement current telemetry technologies. Results for the closely related Canada lynx, which are sympatric in a portion of the bobcat range, indicated all autosomal loci were monomorphic which suggests this panel would remain accurate in areas where the species co-occur.
Data availability
Data from GBS SNP discovery available upon request. All other data have been provided as online supplements.
References
Buchalski MR, Sacks BN, Ahrens KD, Gustafson KD, Rudd JL, Ernest HB et al (2022) Development of a 95 SNP panel to individually genotype mountain lions (Puma concolor) for microfluidic and other genotyping platforms. Conserv Genet Resour 14(2):147–150. https://doi.org/10.1007/s12686-022-01255-6
Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH (2011) Stacks: building and genotyping loci de novo from short-read sequences. G3: Genes, Genomes, Genet 1(3):171–182. https://doi.org/10.1534/g3.111.000240
Creel S, Spong G, Sands JL, Rotella J, Zeigle J, Joe L et al (2003) Population size estimation in Yellowstone wolves with error-prone noninvasive microsatellite genotypes. Mol Ecol 12(7):2003–2009. https://doi.org/10.1046/j.1365-294X.2003.01868.x
Ekblom R, Aronsson M, Elsner-Gearing F, Johansson M, Fountain T, Persson J (2021) Sample identification and pedigree reconstruction in wolverine (Gulo gulo) using SNP genotyping of non-invasive samples. Conserv Genet Resour 13:261–274. https://doi.org/10.1007/s12686-021-01208-5
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES et al (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6(5):e19379. https://doi.org/10.1371/journal.pone.0019379
Herten K, Hestrand MS, Vermeesch JR, Van Houdt JKJ (2015) GBSX: a toolkit for experimental design and demultiplexing genotyping by sequencing experiments. BMC Bioinform 16:73. https://doi.org/10.1186/s12859-015-0514-3
Jombart T, Ahmed I (2011) Adegenet 1.3-1: new tools for the analysis of genome-wide SNP data. Bioinformatics 27(21):3070–3071. https://doi.org/10.1093/bioinformatics/btr521
Kozakiewicz CP, Burridge CP, Funk WC, Salerno PE, Trumbo DR, Gagne RB et al (2019) Urbanization reduces genetic connectivity in bobcats (Lynx rufus) at both intra–and interpopulation spatial scales. Mol Ecol 28(23):5068–5085. https://doi.org/10.1111/mec.15274
Li H (2013) Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv preprint arXiv:1303.3997v1 [q-bio.GN]. https://doi.org/10.48550/arXiv.1303.3997
Piggott M (2004) Effect of sample age and season of collection on the reliability of microsatellite genotyping of faecal DNA. Wildl Res 31(5):485–493. https://doi.org/10.1071/WR03096
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Schultz AJ, Cristescu RH, Littleford-Colquhoun BL, Jaccoud D, Frère CH (2018) Fresh is best: accurate SNP genotyping from Koala scats. Ecol Evol 8(6):3139–3151. https://doi.org/10.1002/ece3.3765
Serieys LE, Lea A, Pollinger JP, Riley SP, Wayne RK (2015) Disease and freeways drive genetic change in urban bobcat populations. Evol Appl 8(1):75–92. https://doi.org/10.1111/eva.12226
Tourvas N (2021) PopGenUtils: a collection of useful functions to deal with genetic data in R. R package version 0.1.6
von Thaden A, Cocchiararo B, Jarausch A, Jüngling H, Karamanlidis A, Tiesmeyer A et al (2017) Assessing SNP genotyping of noninvasively collected wildlife samples using microfluidic arrays. Sci Rep 7:10768. https://doi.org/10.1038/s41598-017-10647-w
von Thaden A, Nowak C, Tiesmeyer A, Reiners TE, Alves PC, Lyons LA et al (2020) Applying genomic data in wildlife monitoring: development guidelines for genotyping degraded samples with reduced single nucleotide polymorphism panels. Mol Ecol Resour 20(3):662–680. https://doi.org/10.1111/1755-0998.13136
Waits LP, Paetkau D (2005) Noninvasive genetic sampling tools for wildlife biologists: a review of applications and recommendations for accurate data collection. J Wildl Manag 69(4):1419–1433. https://doi.org/10.2193/0022-541X(2005)69[1419:NGSTFW]2.0.CO;2
Acknowledgements
G. Edejer, A. Kubicki, C. Kubicki, A. Spicer, and S. Vanderzwan were instrumental in completing the laboratory work. We thank the following for providing Canada lynx samples for our study: M. Flannery and the California Academy of Sciences, Department of Ornithology and Mammalogy (Loan #1612); Colorado Parks and Wildlife; A. Piaggio and the USDA APHIS Wildlife Services, National Wildlife Research Center; D. Straughan, G. Salcido and the U.S. Fish and Wildlife Service, Forensics Laboratory.
Funding
Funding was from the U.S. Fish and Wildlife Service, Wildlife Restoration Act (Grant No. F24AF02292).
Author information
Authors and Affiliations
Contributions
BNS and MRB designed the study; SPQ and KDA performed laboratory work and bioinformatics; BNS discovered the SNPs; BNS and KDA tested and selected the final panel; all authors contributed to writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahrens, K.D., Sacks, B.N., Preckler-Quisquater, S. et al. Development of a 96 SNP panel for fecal genotyping and individual identification of bobcats (Lynx rufus) in California. Conservation Genet Resour (2024). https://doi.org/10.1007/s12686-024-01368-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12686-024-01368-0