Abstract
Finless porpoises (Neophocaena asiaeorientalis) currently face population decline caused by significant human activities and are categorized as endangered on the International Union for Conservation of Nature (IUCN) Red List; however, information on their habitats is currently insufficient. Although conducting visual surveys to determine the distribution of cetaceans is common, visual observation of finless porpoises is challenging owing to their specific morphological and ecological characteristics. In this study, we developed an environmental DNA (eDNA) assay for species-specific detection of finless porpoises. To test the utility of the assay, we conducted a visual survey in parallel with an eDNA survey by collecting water samples from 50 sites throughout Osaka Bay. We visually found a finless porpoise at one location and detected eDNA at nine sites, including sites near the visual observation site and those with rare sightings of finless porpoises. Therefore, in this study, we suggest that the use of eDNA analysis for distribution surveys of finless porpoises will enable more efficient surveys. The proposed eDNA technique can not only be applied to the distribution surveys finless porpoises but also to those of other cetacean species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The finless porpoise (Neophocaena asiaeorientalis) is a cetacean species that belongs to the family Phocoenidae (Amano 2009) (Fig. 1a). It is primarily distributed in the warm waters of the Middle East and Asia, particularly along the coasts of Iran, India, Indonesia, China, Korea, and Japan. In Japan, it is mainly distributed in the following five regions: Sendai to Tokyo Bay, Ise and Mikawa Bays, Seto Inland Sea, Omura Bay, Ariake Sea, and Tachibana Bay (Shirakihara et al. 1992). They form genetically distinct local populations in these areas, as suggested by differences in their mtDNA sequences (Yoshida et al. 2001), breeding seasons (Shirakihara et al. 1993), and skull shapes (Yoshida et al. 1995).
The main known habitat of finless porpoises lies within coastal waters at depths shallower than 50 m (Shirakihara et al. 1994). As a top predator in coastal ecosystems, finless porpoises are known to have a significant impact on the entire food chain in coastal areas. Nevertheless, human activities have threatened the habitat of this species, and its population has declined (Jefferson and Hung 2004; Shirakihara et al. 2007). The eastern part of the Seto Inland Sea, including Osaka Bay, is subject to significant human activity, such as commercial fisheries, vessel traffic, and coastal development, because this area, particularly Osaka Bay, is adjacent to the mega-city of Osaka (Kinki Major Metropolitan Area), with a population of over 19 million (UN 2018). As reported by Kasuya et al. (2002), over a period of 20 years, from the 1970s to 2000, the population of finless porpoises in this area was estimated to have reduced by approximately one-third. Finless porpoises are classified as endangered on the International Union for Conservation of Nature (IUCN) Red List (IUCN 2017), and ongoing human activities in the area have raised concerns regarding their population decline. However, data on the habitat of finless porpoises are insufficient as their visual observation is challenging owing to their morphological and ecological characteristics (Akamatsu et al. 2008; Kimura et al. 2009). Many cetacean species exhibit large body sizes, dorsal fins, and form large pods; thus, visual surveys are a frequently employed method for monitoring their distribution (Swartz et al. 2003; Barlow and Taylor 2005). In contrast, finless porpoises are one of the smallest cetaceans, measuring 1.5 to 2.0 m in length and 50 to 60 kg in weight, and they lack dorsal fins, with only a small portion of their backs visible when they are at the sea surface (Fig. 1b). Additionally, they do not form large pods, and do not exhibit porpoising behavior on the sea surface. For these reasons, visual surveys of finless porpoises are challenging. Therefore, a distribution survey method that is independent of morphology and ecology is crucial.
Environmental DNA (eDNA) analysis for the detection of DNA fragments in the environment, including oceans and inland waters, is an emerging tool for the biomonitoring of underwater species. eDNA surveys have been reported to be simpler and more cost-effective than conventional methods, such as visual and capturing surveys (Burian et al. 2021), and an increasing number of studies have employed eDNA analysis to survey species, especially rare ones that cannot be directly observed through visual surveys (Wada et al. 2020; Bonfil et al. 2021). eDNA analysis can serve as a helpful supplement or possibly a more effective alternative to conventional methods for studying the distribution of finless porpoises. However, studies that have applied eDNA analysis to cetaceans are scarce compared with those to fish, terrestrial mammals, and birds (Foote et al. 2012; Pinfield et al. 2019; Tang et al. 2019; Székely et al. 2021; Robinson et al. 2023). In this study, we aimed to establish an eDNA assay specific to finless porpoises and confirm the capability of eDNA analysis to detect finless porpoise DNA in marine environments.
Materials and methods
Development of a species-specific assay for finless porpoise
The DNA sequences of the mitochondrial cytochrome b (Cytb) gene of the finless porpoise and two other cetacean species identified in Osaka Bay (Indo-Pacific bottlenose dolphin [Tursiops aduncus] and common dolphin [Delphinus delphis]) were downloaded from the National Center for Biotechnology Information (NCBI) (Table S1). Sequences of target and related species were aligned and a single set of primers containing target-specific bases within 5 bases of the 3′ end were designed (Nas-Cytb-F, Nas-Cytb-R) (Table 1). A probe containing target-specific bases was designed using the Primer Express software (version 3.0; Applied Biosystems) with default settings (Nas-Cytb-P) (Table 1).
The specificity of the designed assay was validated in vitro using DNA from target and related species. The DNA of finless porpoise was extracted from muscle tissue preserved in the Suma Marine Aquarium (a by-caught individual in the Harimanada Sea in the eastern part of the Seto Inland Sea), and that of Indo-Pacific bottlenose dolphin was extracted from muscle tissue provided by the Okinawa Churashima Foundation using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Each extracted DNA sample was diluted in 10 mM Tris HCl [pH 8.0] and 1 mM EDTA (TE) buffer at a concentration of 20 pg/µL.
Real-time polymerase chain reaction (PCR) was conducted using a QuantStudio3 real-time PCR system (Thermo Fisher Scientific, Waltham, MA, USA) with muscle-derived DNA as the template. The limit of detection (LOD), which is defined as the lowest concentration of the template for which any of the replicates were positive (Feist et al. 2018; Robinson et al. 2024), was determined using 100 pg/reaction to 1 fg/reaction of the DNA of the target species as the template. Positivity was determined by whether an exponential increase in DNA was observed. The composition of the PCR reaction solution was 1x TaqMan Environmental Master Mix 2.0 (Thermo Fisher Scientific), 900 nM each of primers, 125 nM TaqMan probe, 0.1 µL AmpErace Uracil N-glycosylase (Thermo Fisher Scientific), 5 µL DNA sample, and ultrapure water, with a total volume of 20 µL. PCR blanks were prepared using the same volume of ultrapure water as the DNA samples. Real-time PCR was conducted in four replicates, with an initial step of 50 ℃ for 2 min and 95 ℃ for 10 min, followed by 55 cycles of 95 ℃ for 15 s and 60 ℃ for 1 min.
Field application
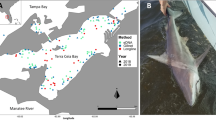
A field survey was conducted on July 25, 2022 to test whether the DNA of finless porpoise could be detected. The survey area was Osaka Bay, and sampling was conducted at 50 sites along a 120-km long line route from Kishiwada Port to Osaka Port through the Kitan Strait, around the Kansai International Airport, and Akashi Strait (Fig. 2). We also conducted a visual survey during the cruise (180° forward; two people standing on the deck; sailing speed: 10–12 kts; weather: clear; and wave height: 0.5 m). To prevent DNA cross-contamination during field survey and lab experiments, disposable gloves were worn and bleached consumables (0.1% effective chlorine concentration) were used. For water sampling, a single 2-L surface water sample was collected at each site, using a bucket, and transferred to a bottle, and a final concentration of 0.01% benzalkonium chloride solution was added to preserve the eDNA (Yamanaka et al. 2017). Moreover, 2 L of purified water was used as the field blank, and a benzalkonium chloride solution was added onboard and then treated in the same manner as the samples. Samples and field blanks were transported to the laboratory in plastic cooler boxes and stored below 10 °C.
The field site of this study is Osaka Bay, Japan. Sampling for eDNA analysis was conducted at 50 sites (white and blue circles) along a 120-km long line route from Kishiwada Port to Osaka Port through the Kitan Strait, around Kansai International Airport, and Akashi Strait. Blue circles represent sites where the eDNA of finless porpoise was detected, and the circle sizes correspond to the number of amplified replicates. The small white circles represent sites where DNA was not detected. The red square represents the site where a finless porpoise was visually observed
The following day, all samples and field blanks were filtered through two glass-fiber filters (GF/F, Cytiva, Tokyo, Japan) with an average pore size of 0.7 μm. The two filters were then pooled and stored at −25 °C until DNA extraction. DNA extraction from the filters was conducted using the DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany) according to the experimental manual published by the eDNA Society (Minamoto et al., 2021) to obtain 100 µL of eDNA samples. Samples were stored at −25 °C until subsequent experiments.
Real-time PCR was conducted on the extracted eDNA samples using the finless porpoise-specific assay. The reagent composition, thermal conditions, and number of replicates for real-time PCR are the same as for the in vitro test described above. Field blanks were treated in the same manner as samples. Positive controls were prepared by adding 5 µL of 0.2 pg/µL of finless porpoise tissue DNA, whereas PCR blanks were prepared by adding the same volume of ultrapure water instead of DNA samples. Real-time PCR amplicons were purified using the Wizard SV Gel and PCR Clean-up System (Promega, Madison, WI, USA) and sent to Fasmac Sequencing Services (Atsugi, Japan) for Sanger sequencing to confirm the sequence of the amplified DNA.
To check for false negatives due to PCR inhibition, real-time PCR with an internal positive control (IPC) was performed. As IPC, 3000 copies/reaction of lambda phage DNA was used. The composition of the PCR reaction solution was 1x TaqMan Environmental Master Mix 2.0, 900 nM each of primers for lambda phage (lambda-7184 F and lambda-7267R; Honjo et al. 2010), 125 nM TaqMan probe (lambda-7210P; Honjo et al. 2010), 0.1 µL AmpErace Uracil N-glycosylase, 5 µL eDNA sample (n = 50) or ultrapure water (n = 3; for positive control), and IPC DNA, in a total volume of 20 µL.
Results
To confirm the specificity of the designed assay, primers and probe sequences were compared with those of closely related species. When compared with common dolphin sequences, there were two or three nucleotide substitutions in the forward primer, two or three nucleotides in the reverse primer and five nucleotide substitutions in the probe. Similarly, with Indo-Pacific bottlenose dolphin, there were three, three or four, and five substitutions in the forward primer, reverse primer, and probe, respectively. For both species, there were substitutions within five bases of the 3′ ends of both primers. These suggest that non-specific amplification of DNA is unlikely for either species.
In vitro specificity testing with tissue-derived DNA confirmed the amplification of finless porpoise DNA, and no amplification of DNA from related species (Indo-Pacific bottlenose dolphin) was observed. PCR blanks showed negative results. The LOD was determined to be 1 fg/reaction, and the PCR efficiency was calculated to be 91.3% based on the Ct values of 10 fg–100 pg/reaction DNA from the LOD experiments.
During the field survey, one finless porpoise was sighted at one site (Fig. 2, red square). In the eDNA analysis, no DNA amplification was observed in the field or PCR blanks, and finless porpoise DNA was detected at nine sites (Fig. 2, blue circles). The areas of detection included Kansai International Airport, near the visual observation site, and the Kitan and Akashi Straits. Of all the detection sites, four, two, and one replicates were positive at one, two, and six sites, respectively (Fig. 2; Table S2). The closest sampling site to where a finless porpoise was observed by visual survey was approximately 250 m away, and no DNA from finless porpoises was detected at the site.
Sanger sequencing and homology searching with Blast showed that all sequences detected in eDNA were identical and showed 97.62–98.81% identity to the finless porpoise sequences in the database. The sequences had no more than 94.05% identity to non-finless porpoise sequences, and were determined to be derived from finless porpoise.
The results of real-time PCR with IPC to confirm the absence of PCR inhibition showed that the cycle threshold (Ct) values of the field samples (n = 50) were 29.55 ± 0.10 (mean ± 1 SD) and the Ct values of the positive controls (n = 3) were 29.73 ± 0.11. There was no difference between the Ct values of the samples and controls, and there was no evidence of PCR inhibition for any of the eDNA samples.
Discussion
We established an eDNA assay specific for finless porpoises and successfully detected eDNA in the field. To our knowledge, this is the first study to detect of finless porpoise DNA in marine environments. Conservation of finless porpoises is an urgent issue that requires immediate attention, and identifying its habitat is a top priority for promoting its conservation. In our study, although finless porpoises were visually confirmed at only one location, their DNA was detected at nine sites, suggesting the effectiveness of eDNA surveys in investigating their distribution.
In this study, water samples were collected from 50 sites, nine of which tested positive for finless porpoise DNA. These results are consistent with the frequent observation of finless porpoises near Kansai International Airport. Three possible explanations exist for the abundance of finless porpoises around Kansai International Airport. First, the water depth in the area is approximately 20 m. Previous studies have reported that finless porpoises prefer water depths shallower than 50 m, particularly those shallower than 20 m (Shirakihara et al. 2007). Therefore, the shallow waters surrounding the airport may be suitable habitat for these species. Second, seaweed beds that have developed on the seawalls of the airport offer habitat for diverse living organisms (Ohno and Serisawa 2002). Finless porpoises, which are opportunistic predators of fish and cephalopods (Amano et al. 2017), may use seaweed beds as feeding grounds, where they can prey on aggregating animals. Third, the area around the airport is designated as a protected area with minimal vessel traffic. Finless porpoises do not appear to show a preference for vessel traffic, making the area around the airport a potentially favorable environment.
eDNA was also detected at locations with rare sighting records of finless porpoises, such as near the Kitan and Akashi Straits, where the average water depth was approximately 70 m. This result suggests that finless porpoises inhabit areas with a water depth of 70 m, although they prefer to inhabit areas with water depths shallower than 50 m.
Although a single finless porpoise was sighted at one site during the visual survey, its eDNA was not detected at the sampling site, which was approximately 250 m from the observation point. Murakami et al. (2019) suggested that the dispersion area of eDNA in the ocean is only approximately 30 m. Thus, for eDNA analysis, finer-scale sampling is necessary to accurately determine its distribution. However, considering the detection of eDNA at locations without the visual observation of finless porpoises, we suggest the use of eDNA analysis as a complementary method to visual surveys to assess their distribution.
Finless porpoises typically favor coastal areas; however, eDNA was not detected in the coastal areas of Hanshin (Fig. 2). The coastal area of Hanshin is home to vast ports of Osaka and Kobe (Fig. 2), with heavy vessel traffic (Gao and Shiotani 2013). Finless porpoises are extremely vigilant and favor environments with low vessel traffic. Akamatsu et al. (2008) recorded the vocalizations of finless porpoises using a stereo acoustic event recorder and reported that most finless porpoises were detected at night, as opposed to during hours of heavy vessel traffic between 12:00 AM and 6:00 PM, and that the pattern of presence was possibly driven by vessel traffic. Thus, the results of eDNA detection reflect this behavior, and the Hanshin area may not be a suitable habitat for finless porpoises.
One weakness of our study is that in silico and in vitro tests have only been conducted on a limited number of species observed in Osaka Bay. In order to apply the assay designed in this study to other areas inhabited by finless porpoises, it is necessary to verify their specificity for sympatric species in each area. However, in the present study, sequencing of amplicons derived from eDNA confirms that they are certainly finless porpoise DNA, and therefore, the above weaknesses do not affect the results of this study.
Environmental DNA survey is expected to complement conventional methods and aid in the conservation of finless porpoises by quickly and accurately monitoring their distribution. In the future, the type of habitat preferred by finless porpoises and the extent of influence of human activities can be determined by comparing their distribution data obtained through eDNA analysis with the surrounding environmental data, including water depth, type of sea bottom, water quality, presence of seaweed beds, and vessel traffic. Additionally, more frequent surveys to determine the seasonal changes in their distribution would help clarify their ecology, including whether they have migrated into and out of Osaka Bay.
Furthermore, considering that eDNA analysis is a useful tool for estimating intraspecific genetic diversity (Parsons et al. 2018; Székely et al. 2021), it can be applied to assess the genetic diversity of local finless porpoise populations for conservation purposes. This study presents a unique application of eDNA analysis for cetaceans.
Conventional distribution survey techniques for cetaceans, such as visual surveys, have challenges, such as restricted survey areas and survey difficulties resulting from sea conditions (Mellinger and Barlow 2003). Recently, visual surveys have been conducted using unmanned aerial vehicles (UAVs) (Hodgson et al. 2013); however, the use of UAVs is restricted to the vicinity of the airport and cannot be used in our study area. eDNA analysis overcomes these issues and provides the advantage of determining the distribution of other organisms after sample collection. Therefore, eDNA analysis can be used for other cetaceans, particularly those that cannot easily be observed by visual surveys or bio-logging. Additionally, the proposed method can not only be used for distribution surveys but also for obtaining novel insights into the ecology of the target species by studying the distribution and surrounding biological environment across a vast expanse.
Data availability
No datasets were generated or analysed during the current study.
References
Amano M (2009) Finless porpoise: Neophocaena phocaenoides In Encyclopedia of marine mammals (pp. 437–439). Academic Press. https://doi.org/10.1016/B978-0-12-373553-9.00103-6
Amano M, Kusumoto M, Abe M, Akamatsu T (2017) Long-term effectiveness of pingers on a small population of finless porpoises in Japan. Endanger Species Res. https://doi.org/10.3354/esr00776
Akamatsu T, Nakazawa I, Tsuchiyama T, Kimura N (2008) Evidence of nighttime movement of finless porpoises through Kanmon Strait monitored using a stationary acoustic recording device. Fish Sci. https://doi.org/10.1111/j.1444-2906.2008.01614.x
Barlow J, Taylor BL (2005) Estimates of sperm whale abundance in the northeastern temperate Pacific from a combined acoustic and visual survey. Mar Mamm Sci. https://doi.org/10.1111/j.1748-7692.2005.tb01242.x
Bonfil R, Palacios-Barreto P, Vargas OUM, Ricaño-Soriano M, Díaz-Jaimes P (2021) Detection of critically endangered marine species with dwindling populations in the wild using eDNA gives hope for sawfishes. Mar Biol 168(5):60. https://doi.org/10.1007/s00227-021-03862-7
Burian A, Mauvisseau Q, Bulling M, Domisch S, Qian S, Sweet M (2021) Improving the reliability of eDNA data interpretation. Mol Ecol Resour 21(5):1422–1433. https://doi.org/10.1111/1755-0998.13367
Feist SM, Jones RL, Copley JL, Pearson LS, Berry GA, Qualls CP (2018) Development and validation of an environmental DNA method for detection of the alligator snapping turtle (Macrochelys temminckii). Chelonian Conserv Biology 17(2):271–279. https://doi.org/10.2744/CCB-1315.1
Foote AD, Thomsen PF, Sveegaard S, Wahlberg M, Kielgast J, Kyhn LA, Salling AB, Galatius A, Orlando L, Gilbert MTP (2012) Investigating the potential use of environmental DNA (eDNA) for genetic monitoring of marine mammals. PLoS ONE 7(8):e41781 https://doi.org/10.1371/journal.pone.0041781
Gao X, Shiotani S (2013), September An effective presentation of navigation information for prevention of maritime disaster using AIS and 3D-GIS. In 2013 OCEANS-San Diego (pp. 1–6). IEEE. https://doi.org/0.23919/OCEANS.2013.6741127
Hodgson A, Kelly N, Peel D (2013) Unmanned aerial vehicles (UAVs) for surveying marine fauna: a dugong case study. PLoS ONE 8(11):e79556. https://doi.org/10.1371/journal.pone.0079556
Honjo MN, Minamoto T, Matsui K, Uchii K, Yamanaka H, Suzuki AA, Kohmatsu Y, Iida T, Kawabata Z (2010) Quantification of Cyprinid herpesvirus 3 in environmental water by using an external standard virus. Appl Environ Microbiol 76(1):161–168. https://doi.org/10.1128/AEM.02011-09
Jefferson TA, Hung SK (2004) Neophocaena phocaenoides. Mammalian Species 746:1–12.
Kasuya T, Yamamoto Y, Iwatsuki T (2002) Abundance decline in the finless porpoise population in the Inland Sea of Japan. Raffles Bulletin of Zoology 10: 57–65.
Kimura S, Akamatsu T, Wang K, Wang D, Li S, Dong S, Arai N (2009) Comparison of stationary acoustic monitoring and visual observation of finless porpoises. J Acoust Soc Am. https://doi.org/10.1121/1.3021302
Mellinger D, Barlow J (2003) Future directions for acoustic marine mammal surveys: stock assessment and habitat use. Report of a workshop held in La Jolla. CA, p 37. 20–22 November 2002, NOAA OAR Special Report, NOAA/PMEL Contribution No. 2557
Minamoto M Sado, Seino, Doi, Kondoh, Nakamura, Takahara, Yamamoto, Yamanaka, Araki, Iwasaki, Kasai, Masuda & Uchii (2021) An illustrated manual for environmental DNA research: water sampling guidelines and experimental protocols. Environ DNA 3(1):8–13 https://doi.org/10.1002/edn3.121
Murakami H, Yoon S, Kasai A, Minamoto T, Yamamoto S, Sakata MK, Horiuchi T, Sawada H, Kondoh M, Yamashita Y, Masuda R (2019) Dispersion and degradation of environmental DNA from caged fish in a marine environment. Fish Sci 85:327–337. https://doi.org/10.1007/s12562-018-1282-6
Ohno M, Serisawa Y (2002) Recent reports on seaweed and seagrass establishment and restoration. Fish Sci 68(sup2):1737–1742. https://doi.org/10.2331/fishsci.68.sup2_1737
Parsons KM, Everett M, Dahlheim M, Park L (2018) Water, water everywhere: environmental DNA can unlock population structure in elusive marine species. Royal Soc open Sci 5(8):180537. https://doi.org/10.1098/rsos.180537
Pinfield R, Dillane E, Runge AKW, Evans A, Mirimin L, Niemann J, Reed TE, Reid DG, Rogan E, Samarra FIP, Sigsgaard EE, Foote AD (2019) False-negative detections from environmental DNA collected in the presence of large numbers of killer whales (Orcinus orca). Environ DNA 1(4):316–328. https://doi.org/10.1002/edn3.32
Robinson CV, Dracott K, Glover RD, Warner A, Migneault A (2024) DNA from dives: species detection of humpback whales (Megaptera novaeangliae) from flukeprint eDNA. Environ DNA 6(2):e524. https://doi.org/10.1002/edn3.524
Robinson CV, Migneault A, Dracott K, Glover RD (2023) Seas the DNA? Limited detection of cetaceans by low-volume environmental DNA transect surveys. Environ DNA. https://doi.org/10.1002/edn3.485
Shirakihara K, Shirakihara M, Takemura A (1994) Distribution and seasonal density of the finless porpoise Neophocaena phocaenoides in the coastal waters of western Kyushu, Japan. Fish Sci. https://doi.org/10.2331/fishsci.60.41
Shirakihara K, Shirakihara M, Yamamoto Y (2007) Distribution and abundance of finless porpoise in the Inland Sea of Japan. Mar Biol 150:1025–1032. https://doi.org/10.1007/s00227-006-0363-8
Shirakihara M, Takemura A, Shirakihara K (1993) Age, growth, and reproduction of the finless porpoise, Neophocaena phocaenoides, in the coastal waters of western Kyushu, Japan. Mar Mamm Sci 9(4):392–406. https://doi.org/10.1111/j.1748-7692.1993.tb00472.x
Shirakihara K, Yoshida H, Shirakihara M, Takemura A (1992) A questionnaire survey on the distribution of the finless porpoise, Neophocaena phocaenoides, in Japanese waters. Mar Mamm Sci 8(2):160–164. https://doi.org/10.1111/j.1748-7692.1992.tb00376.x
Swartz SL, Cole T, McDonald MA, Hildebrand JA, Oleson EM, Martinez, Martinez A, Clapham PJ, Barlow. J., Jones ML (2003) Acoustic and visual survey of humpback whale (Megaptera novaeangliae) distribution in the eastern and southeastern Caribbean Sea. Caribb J Sci 39(2):195–208
Székely D, Corfixen NL, Mørch LL, Knudsen SW, McCarthy ML, Teilmann J, Heide-Jørgensen MP, Olsen MT (2021) Environmental DNA captures the genetic diversity of bowhead whales (Balaena mysticetus) in West Greenland. Environ DNA 3(1):248–260. https://doi.org/10.1002/edn3.176
Tang Y, Wu Y, Liu K, Li J, Li H, Wang Q, Xu P (2019) Investigating the distribution of the Yangtze finless porpoise in the Yangtze River using environmental DNA. PLoS ONE 14(8):e0221120. https://doi.org/10.1371/journal.pone.0221120
The IUCN red list of threatened species Version (2017) 3.1. http://www.iucnredlist.org (2017). Accessed on 05 November 2023
UNDESA P (2018) World urbanization prospects: the 2018 revision. Retrieved August, 26, 2018
Wada T, Doi H, Togaki D, Kaida R, Nagano M, Katano I, Suzuki M, Ohtani T, Mitsuhashi H (2020) Exploring a legendary giant squid: an environmental DNA approach. Mar Biol 167:1–6. https://doi.org/10.1007/s00227-020-03773-z
Yamanaka H, Minamoto T, Matsuura J, Sakurai S, Tsuji S, Motozawa H, Hongo M, Sogo Y, Kakimi N, Teramura I, Sugita M, Baba M, Kondo A (2017) A simple method for preserving environmental DNA in water samples at ambient temperature by addition of cationic surfactant. Limnology 18:233–241. https://doi.org/10.1007/s10201-016-0508-5
Yoshida H, Shirakihara K, Shirakihara M, Takemura A (1995) Geographic variation in the skull morphology of the finless porpoise Neophocaena phocaenoides in Japan waters. Fish Sci 61(4):555–558. https://doi.org/10.2331/fishsci.61.555
Yoshida H, Yoshioka M, Shirakihara M, Chow S (2001) Population structure of finless porpoises (Neophocaena phocaenoides) in coastal waters of Japan based on mitochondrial DNA sequences. J Mammal 82(1):123–130. https://doi.org/10.1644/1545-1542(2001)082%3C0123:PSOFPN%3E2.0.CO;2
Acknowledgements
We would like to thank Kyouhei Senpaku Co., LTD, Dr. Qianqian Wu, Mr. Itsuki Hirayama, and Ms. Niko Nakamura (Graduate School of Human Development and Environment, Kobe University) for their help with seawater sampling, and the Okinawa Churashima Foundation for providing the tissue samples. This study was financially supported by the Organization of Advanced and Integrated Research, Kobe University, and the Environment Research and Technology Development Fund (JPMEERF20224R01) of the Environmental Restoration and Conservation Agency, provided by the Ministry of the Environment of Japan.
Funding
Open Access funding provided by Kobe University.
Author information
Authors and Affiliations
Contributions
N.H., T.I., and T.M. conceived the study design; N.H. and T.I. conducted the field survey; N.H., N.K. and M.K.S. performed laboratory experiments; K.N. collected tissue sample. N.H. and T.M. lead to write and edit the manuscript; All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Toshifumi Minamoto is an inventor of the patent for the use of benzalkonium chloride for eDNA preservation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hashimoto, N., Iwata, T., Kihara, N. et al. Detection of environmental DNA of finless porpoise (Neophocaena asiaeorientalis) in Osaka Bay, Japan. Conservation Genet Resour (2024). https://doi.org/10.1007/s12686-024-01361-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12686-024-01361-7