Abstract
Metal-organic cobalt hydroxide emerges as a cost-effective electrocatalyst for the oxygen evolution reaction (OER) in energy conversion. However, the limited active sites and poor conductivity hinder their large-scale application. This study employed salicylate as a bridging ligand to synthesize iron-incorporated metal-organic cobalt hydroxide. The influence of Fe intercalation on Co(OH)(Hsal) (where Hsal denotes o-HOC6H4COO−) was investigated using X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). Fe0.2Co0.8(OH)(Hsal) demonstrates remarkable electrocatalytic activity, displaying an OER overpotential of 298 mV at 10 mA cm−2 and a Tafel slope of 57.46 mV dec−1. This enhancement can be attributed to improved charge transfer kinetics and increased active sites. This work highlights the crucial role of Fe in improving the efficiency of Co-based oxygen-evolving catalysts (OECs) and its potential for boosting efficient hydrogen generation in alkaline environments.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The consumption of traditional energy sources and environmental concerns about pollution have driven the exploration of renewable and eco-friendly energy solutions to the forefront of scientific research. Within this context, water-splitting technology has gained significant attention, offering the opportunity to convert renewable energy sources, including solar, wind, and tidal energy, into carbon-neutral energy carriers, such as hydrogen. Despite its immense potential, water-splitting technology confronts a substantial challenge: the sluggish kinetics of the oxygen evolution reaction (OER) hinders overall reaction efficiency [1]. To address this challenge, researchers focus their efforts on catalyst development, including optimizing the catalytic active sites, improving the electron transfer properties of the catalyst, enhancing surface oxygen adsorption capacity, and modulating the crystal structure of the catalyst [2, 3]. Through exploring various methods to enhance catalysts, researchers have discovered the remarkable potential of transition metal-derived catalysts, prompting a shift in interest from traditional noble metal catalysts toward cost-effective alternatives such as cobalt (Co), iron (Fe), nickel (Ni), manganese (Mn), and copper (Cu) [4].
Compared with common oxygen evolution catalysts such as transition metal oxides and hydroxides, metal-organic frameworks (MOFs) have attracted great attention due to their periodic structure, evenly dispersed metal centers, and high specific surface area in electrocatalysis [5]. However, single MOFs usually exhibit limited OER activity because of the constant electronic structure of the catalytic center [6]. Defect engineering [7] has been recognized as a potential way to modify the active site coordination environment, thereby optimizing the reaction kinetics. He et al. [8] reported a Co80Fe20(OH)(OCH3) (CoFeMe) with rich vacancies synthesized by a cation exsolution process in a one-step solve-thermal reaction. The introduction of intercalation, Fe integration, and vacancies synergistically reduces the overpotential of CoFeMe to 240 mV at a current density of 10 mA cm−2. This substantial improvement of 110 mV demonstrates the significant impact of these modifications. Moreover, modification of the electronic structure of the active site is also a strategy to improve the activity of catalysts. For example, Xue et al. [9] synthesized missing-linker MOFs for OER, and these missing-linker MOFs have modified the cobalt coordination environment by changing the charge distribution. As a result, it shows an excellent OER performance with an ultralow overpotential of 241 mV at 100 mA cm−2. However, those conventional synthetic methods often require costly reagents such as organic solvents, organometallic precursors, surfactants, and modulators or involve complexed procedures and equipment. Hence, there is a growing emphasis on seeking economical and universally applicable synthesis approaches while integrating dual transition metal catalysis.
In this study, we present a simple, cost-effective, and scalable synthesis method for M-incorporated metal-organic cobalt hydroxide (MxCo1-x(OH)(Hsal) (where Hsal stands for o-HOC6H4COO−) under mild conditions. The catalyst is synthesized without the need for complex surfactants or regulators. XPS and operando electrochemical impedance spectroscopy (ESI) tests reveal that Fe incorporation introduces abundant vacancies, altering the surface electronic structure. As a result, Fe0.2Co0.8(OH)(Hsal) exhibits an overpotential of only 298 mV to achieve a current density of 10 mA cm−2, displaying a 74 mV reduction compared to the original Co(OH)(Hsal) (372 mV). This work proposes Fe0.2Co0.8(OH)(Hsal) as a highly efficient catalyst and provides more opportunities for its widespread application in OER.
Materials and Methods
Chemicals
Fe(NO3)2·9H2O, Co(NO3)2·6H2O, Ni(NO3)2·6H2O, Cu(NO3)2·6H2O, MnCl2·4H2O, and Nafion solution (5 wt%) were bought from Sigma-Aldrich. Sodium salicylate (NaHsal) and NiCl2·6H2O (98%) were purchased from Alfa Aesar. KOH (99.98%) was purchased from Merck KGaA. All chemicals were used directly without further purification. Deionized (DI) water with a resistivity of 18 MΩ from the Millipore system was used in the experiments.
Synthesis of MxCo1-x(OH)(Hsal) (M = Fe, Ni, Cu, and Mn)
The synthesis of M0.2Co0.8(OH)(Hsal) with the incorporation of various metals (Fe, Ni, Cu, and Mn) was conducted following a procedure outlined in a previous work [10]. Typically, a solution containing 10 mL of Co(NO3)2·6H2O (0.8 mmol) and other metal salts (0.2 mmol, Fe(NO3)2·9H2O, Ni(NO3)2·6H2O, Cu(NO3)2·6H2O, or MnCl2·4H2O) was mixed with a 15 mL aqueous NaHsal solution (4 mmol) under continuous stirring. Maintaining a pH of 7 was achieved using a 0.5 M NaOH solution. The reaction was carried out at 75 °C for 5 h, and subsequently, the products were separated via centrifugation, washed with DI water, and dried at 60 °C in air. These resulting products were denoted as M0.2Co0.8(OH)(Hsal) (M = Fe, Ni, Mn, Cu).
For synthesizing MxCo1-x(OH)(Hsal), the same procedure was followed as mentioned above. However, we systematically adjusted the Fe/Co ratio to achieve compositions of 5/95, 10/90, 20/80, and 30/70. Consequently, the products were denoted as Fe0.05Co0.95(OH)(Hsal), Fe0.1Co0.9(OH)(Hsal), Fe0.2Co0.8(OH)(Hsal), and Fe0.3Co0.7(OH)(Hsal), respectively.
Characterization
Transmission electron microscope (TEM) images were acquired using a JEOL 3000F instrument operating at 300 kV. Additionally, energy-dispersive X-ray spectroscopy (EDS) mapping was performed with the Oxford X-Max EDS detector. X-ray diffraction (XRD) analysis was performed utilizing a Rigaku MiniFlex instrument under the following operational parameters: 40 kV and 15 mA, employing Cu Kα radiation with a wavelength (λ) of 1.541 Å. X-ray photoelectron spectroscopy (XPS) data were acquired on the Thermo Fisher Scientific Nexsa XPS instrument, equipped with Al Kα radiation. The binding energies obtained during XPS analysis were corrected for specimen charging, referencing the C 1s peak to 284.8 eV. Elemental composition analysis was conducted using inductively coupled plasma mass spectrometry (ICP-MS).
Electrochemical Measurements
The electrocatalytic performance of the catalysts was evaluated using a Biologic SP300 electrochemical workstation within a standard three-electrode system. The counter electrode consisted of a graphite rod, while a Hg/HgO electrode, saturated in N2-saturated with a 1 mol L-1 KOH solution, served as the reference electrode. The catalyst ink was prepared through a methodical sequence. First, 1-mg catalysts were mixed with 100-µL DI water, 140 µL ethanol, and 10 µL of 5 wt% Nafion solution. This mixture was subjected to ultrasonication for 1 h. Second, 5 µL of the resulting ink was uniformly dispersed onto a glassy carbon electrode polished with Al2O3 (diameter = 0.5 cm), which served as the working electrode (WE) with a catalyst loading mass of 0.102 mg cm-2. Subsequently, the WE can be further used after drying at room temperature. The same recipe was followed for the control sample of 20 wt% Pt/C.
Before operating the oxygen evolution reaction (OER) tests, the optimization of the WE involved cyclic voltammetry (CV) scans ranging from 0.2 to − 0.8 V vs. Hg/HgO at a scan rate of 25 mV s-1 until achieving stability. Linear sweep voltammetry (LSV) plots were generated at scan rates of 10 mV s-1 in a 1 mol L-1 KOH solution, maintaining the process until stability was attained. Polarization curves were depicted as overpotential (η) against the logarithm of current (log[J]) to extract Tafel plots. The Tafel slope (b) was determined by fitting the linear segment of the Tafel plots to the Tafel equation (η = b log[J] + a). EIS was acquired under open-circuit voltage conditions across a frequency range from 105 Hz to 5 mHz, employing an amplitude of 10 mV s-1. Moreover, stability tests were conducted using chronoamperometry (CP) at a consistent current density of 10 mA cm-2. All the potential of the reference hydrogen electrode (RHE) was normalized using the following equation: ERHE = EHg/Hgo + 0.098 + 0.059 pH. The typical specific capacitance for a flat surface usually falls between 20 and 60 μF cm−2. In this analysis, a value of 40 μF cm−2 was applied to determine the electrochemically active surface area.
Results and Discussion
Morphology Characterizations of Fe0.2Co0.8(OH)(Hsal)
Figure 1a demonstrates a one-step hydrothermal process used to synthesize MxCo1-x(OH)(Hsal). Salicylates serve as strong organic linkers, displaying strong coordination with metal ions [11]. The environment was maintained at neutral conditions while adjusting the mixture by NaOH to keep the phenolic salicylate in its non-ionized state (pKa2 13.5) [12]. The obtained final product was named Fe0.2Co0.8(OH)(Hsal). The TEM image in Fig. 1b illustrates a flaky, wrinkled structure of Fe0.2Co0.8(OH)(Hsal). High-resolution transmission electron microscopy (HRTEM) revealed the amorphous nature of the formed samples (Fig. 1c, d), which can be potentially attributed to water intercalation and the low synthesis temperature. Moreover, the elemental mapping images (Fig. 1e–h) of C, O, Co, and Fe distinctly demonstrate the uniform distribution of the inserted Fe element within the Fe0.2Co0.8(OH)(Hsal). In addition, the sample also exhibits good porosity, as shown in Fig. S4; Fe0.2Co0.8(OH)(Hsal) has a surface area of 215.92 m2/g.
Structure Characterizations of Fe0.2Co0.8(OH)(Hsal) and Control Samples
Figure 2a shows the specific proportion of Fe in each sample based on the ICP-MS results. The Fe content in FexCo1-x(OH)(Hsal) (X = 0.05, 0.1, 0.2, 0.3) has a specific value of 5.6%/9.5%/21%/30%. These results are close to the theoretical ratio. Figure 2b illustrates the FT-IR spectrum of Co(OH)(Hsal) and Fe0.2Co0.8(OH)(Hsal). The distinctive asymmetries and symmetries centered at 1598/1576 cm−1 and 1467/1443 cm−1 [13] belong to COO− bands, respectively. The presence of the COO− bands confirms the coordination of the carboxylate group with metal centers. After a certain amount of Fe is introduced into the coordination, the characteristic broad peak of the COO− band can still be observed in Fe0.2Co0.8(OH)(Hsal). Additionally, a broad band centered at 3418 cm−1 indicates the existence of water molecules and extensive hydrogen bonding. Furthermore, the broad absorption at 3627 cm−1 corresponds to the O-H stretching mode of the Co-OH groups, akin to observations in layered cobalt hydroxide [14]. The peak of the metal-oxygen vibration (M-O) within the 500–640 cm−1 range signifies the coordination of M-O [15].
a The specific proportion of Co/Fe based on the ICP-MS; b FT-IR spectra and c XRD patterns of Co(OH)(Hsal), Fe0.2Co0.8(OH)(Hsal), and Fe(OH)(Hsal), respectively; d XPS full scan spectrum of Fe0.2Co0.8(OH)(Hsal); e Co 2p spectrum of Co(OH)(Hsal) and Fe0.2Co0.8(OH)(Hsal) before and after the optimization test; f O 1s spectrum of Co(OH)(Hsal) and Fe0.2Co0.8(OH)(Hsal)
The structures of Fe0.2Co0.8(OH)(Hsal) and control samples were characterized by XRD. In Fig. 2c, Co(OH)(Hsal) shows a strong peak at about 7.12° and a peak at 14.24°, which is consistent with the basal reflection and its second harmonics of a typical layer structure [16]. Co(OH)(Hsal) have a low crystallinity compared with the reported work [17] due to the low hydrothermal temperature. Moreover, after Fe is introduced, the peak of Fe0.2Co0.8(OH)(Hsal) at 7.12° disappeared, which indicates that the final product tends to be amorphous, which is consistent with the TEM results.
To further explore the electronic states of Co after Fe incorporation, the Co 2p XPS spectrum of Co(OH)(Hsal) was initially analyzed (Fig. 2e). The resolved peaks at 796.6 eV and 781.2 eV were attributed to Co 2p1/2 and 2p3/2, respectively. The peaks at 803.2 eV and 786.2 eV corresponded to their satellite peaks. Upon the introduction of 20% Fe, the Co 2p1/2 and 2p3/2 positions in the Fe0.2Co0.8(OH)(Hsal) sample exhibited a negative shift of 0.5 eV relative to those binding energies in Co(OH)(Hsal). This shift in energy levels signifies the modulation of the electronic structure of Co due to Fe intercalation [18]. The Co peak shift was also noted during the CV activation process, and a 0.6 eV negative shift was observed after 20 optimization cycles, suggesting ongoing electronic structure changes of Co until it settled. This corresponds with the stable CV performance after 20 cycles in Fig. 3c. However, it can be seen from Fig. S2 that the structure of FT-IR and XRD did not change much after CV activation. This means that after CV activation, the structure and functional group structure of the material did not change greatly. Figure 2f illustrates the O 1s XPS spectrum. The peaks detected at 529.38 eV, 531.58 eV, and 532.58 eV correspond to metal-oxygen (M-O) bonds, M-OH bonds within Co(OH)(Hsal), and adsorbed H2O. Upon Fe incorporation, negative shifts of 0.2 and 0.1 eV are observed in the M-O and M-OH peaks, respectively. The increased M-O ratio due to Fe intercalation supports the generation of the Co-O structure, a vital intermediate facilitating the oxygen evolution reaction [19].
a The LSV curves. b EIS curves of MxCo1-x(OH)(Hsal). c The CV curves of Mn0.2Co0.8(OH)(Hsal) and Fe0.2Co0.8(OH)(Hsal). d The LSV curves of FexCo1-x(OH)(Hsal) (x = 0, 0.05, 0.1, 0.2, 0.3, and 1). e The LSV curves of Co(OH)(Hsal), Fe0.2Co0.8(OH)(Hsal), and RuO2 samples in 1 M KOH solution. f The corresponding Tafel plots of as-prepared
Electrochemical Performance of Oxygen Evolution Reaction
The OER activity of M-incorporated cobalt catalysts is assessed in a standard three-electrode setup in 1 M KOH. Figure 3a reveals the activity of the catalyst when the Fe, Ni, Mn, and Cu proportions are at 20%. Notably, Fe significantly alters the redox electrochemistry of Co, evident from the oxidation peak shift in the low-voltage region (1.0 ~ 1.1 V) compared to pure Co(OH)(Hsal). Figure 3b illustrates the smaller Rct value of Fe0.2Co0.8(OH)(Hsal) sample (4.9 Ω) compared to that of Ni0.2Co0.8(OH)(Hsal) (25.2 Ω), Mn0.2Co0.8(OH)(Hsal) (29.8Ω), Cu0.2Co0.8(OH)(Hsal) (32.1 Ω), and Co(OH)(Hsal) (31.7 Ω), indicating faster reaction kinetics of Fe0.2Co0.8(OH)(Hsal). The stable CV curve in Fig. 3c exhibits an increasing reactivity trend for Fe0.2Co0.8(OH)(Hsal) with increasing cycles [20], while Mn0.2Co0.8(OH)(Hsal) gradually decreases due to possible structure changes or deactivation during the multiple cycles. As shown in Fig. S3, there is a voltage peak shift in the negative direction between 1.1 and 1.2 V (vs. RHE), indicating that Co gradually moves to a lower oxidation state, which is consistent with the XPS result of Fig. 2e. In summary, by affecting the redox electrochemistry of Co and the continuous activation of the electrode, Fe maximizes the catalytic performance and stability of MxCo1-x(OH)(Hsal).
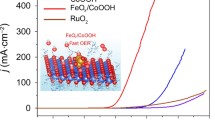
Figure 3d and e demonstrates variations in the LSV curve of FexCo1-x(OH)(Hsal) samples with increasing Fe content. The overpotential decreases from 372 to 298 mV as Fe content reaches 20%, yet rises to 327 mV at 30%. The best performance enhancement for FexCo1-x(OH)(Hsal) is observed at approximately 20% Fe content. Notably, Fe0.2Co0.8(OH)(Hsal) outperforms commercial RuO2 [21], requiring only 298 mV overpotential at a current density of 10 mA cm−2 in N2-saturated 1 M KOH electrolyte, exceeding RuO2 (343 mV). Tafel diagrams extracted from the LSV curve (Fig. 3f) display lower fitted Tafel slope values for Fe0.2Co0.8(OH)(Hsal) (57.46 mV dec−1) compared to commercial RuO2 (108.58 mV dec−1) and Co(OH)(Hsal) (95.69 mVdec−1), indicating promoted OER kinetics and higher activity in Fe0.2Co0.8(OH)(Hsal).
Operando EIS Investigation
To explore the charge transfer kinetics of both Co(OH)(Hsal) and Fe0.2Co0.8(OH)(Hsal) samples, we conducted EIS measurements. Notably, when the applied potential is 1.55 V versus RHE, the charge transfer resistance (Rct) of Fe0.2Co0.8(OH)(Hsal) (4.6Ω) significantly contrasts with that of Co(OH)(Hsal) (68.2Ω) (Fig. 4a), suggesting a remarkably accelerated charge transfer rate in Fe0.2Co0.8(OH)(Hsal) relative to Co(OH)(Hsal). In situ electrochemical impedance spectroscopy (operando EIS) was conducted at varied voltages to explore electron transfer resistance and electrocatalytic kinetics during the OER process. Figure 4b shows the behavior of Co(OH)(Hsal) within the low voltage range (1.3 to 1.5 V versus RHE), displaying a vertically oriented Nyquist curve aligned with the y-axis, indicative of capacitive behavior. The distinct semicircle that emerges above 1.55 V signifies the onset of electrocatalytic OER. Notably, Fe0.2Co0.8(OH)(Hsal) exhibits an initial OER potential of 1.5 V (Fig. 4c). Additionally, the Bode phase plot diagram illustrates the phase angle’s (θ) frequency-dependent variation. Typically, the peak phase angle in the high- and low-frequency ranges correlates with electron conduction and interface reaction charge transfer within the inner layer of the catalyst. Within the medium frequency range (10–1000 Hz) at an open-circuit voltage of 1.3 V, Fe0.2Co0.8(OH)(Hsal) displays significantly reduced impedance compared to Co(OH)(Hsal). This reduction is associated with the increased accessibility of OH− migrating to the surface [22]. Additionally, with increasing applied potential, Fe0.2Co0.8(OH)(Hsal) exhibits a faster decrease in phase angle compared to Co(OH)(Hsal), indicated by the dotted arrows in Fig. 4d and e, specifically. Within the potential range of 1.5 to 1.6 V and the low-frequency region (0.1–10 Hz), the reduced phase angle of Fe0.2Co0.8(OH)(Hsal) suggests enhanced interface reaction charge transfer, particularly beneficial due to Fe intercalated, facilitating faster OER kinetics [23]. Within the identical voltage range, Fe0.2Co0.8(OH)(Hsal) exhibits an elevated ECSA of 15.89 mF cm−2 compared to Co (OH)(Hsal) (6.87 mF cm−2) in Fig. S5, further emphasizing an increased availability of active sites for the OER dynamic process. To explore the inherent activity of the samples, the apparent current density was additionally adjusted relative to their corresponding ECSA. The normalization plots in Fig. S6 illustrate that the OER activity of Fe0.2Co0.8(OH)(Hsal) significantly surpasses that of Co(OH)(Hsal).
a Nyquist plots (at 1.55 V versus RHE) of Co(OH)(Hsal) and Fe0.2Co0.8(OH)(Hsal). b, c Nyquist plots measured for Co(OH)(Hsal) and Fe0.2Co0.8(OH)(Hsal) at various potentials in 1 M KOH. d, e Corresponding Bode plots. f Phase peak angles of Co(OH)(Hsal) and Fe0.2Co0.8(OH)(Hsal) at 1.50–1.60 V versus RHE
Conclusions
In summary, the Fe-induced incorporation method in Co(OH)(Hsal) significantly boosts the OER activity of the catalyst. This method combines element incorporation, intercalation, and defect engineering hydrothermally in a single step, using affordable chemicals and gentle temperatures for synthesis. The resulting Fe0.2Co0.8(OH)(Hsal) catalyst demonstrates exceptional OER electrocatalytic performance, featuring a low potential of 298 mV at a current density of 10 mA cm−2 which is 74 mV lower than Co(OH)(Hsal), and the corresponding Tafel slope is 57.46 mVdec−1. The incorporation of Fe in Fe0.2Co0.8(OH)(Hsal) enriches the catalyst with more active sites, augments intrinsic activity, and enhances charge transfer kinetics, thereby establishing efficient OER activity. Furthermore, the broad applicability of this synthesis method was further validated by successfully developing organic Co hydroxides substituted by three other transition metals (M = Ni, Mn, and Cu) and subjecting them to OER testing. This study lays the groundwork for constructing cost-effective and efficient organic transition metal hydroxide catalysts, offering a new concept and synthesis methods for the design of OER catalysts.
Data Availability
No datasets were generated or analyzed during the current study.
References
N.T. Suen, S.F. Hung, Q. Quan, N. Zhang, Y.J. Xu, H.M. Chen, Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 46, 337–365 (2017)
D.Y. Kuo, J.K. Kawasaki, J.N. Nelson, J. Kloppenburg, G. Hautier, K.M. Shen, D.G. Schlom, J. Suntivich, Influence of surface adsorption on the oxygen evolution reaction on IrO(2)(110). J. Am. Chem. Soc. 139, 3473–3479 (2017)
Y. Sun, Z. Xue, Q. Liu, Y. Jia, Y. Li, K. Liu, Y. Lin, M. Liu, G. Li, C.Y. Su, Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 12, 1369 (2021)
M. Tahir, L. Pan, F. Idrees, X. Zhang, L. Wang, J.-J. Zou, Z.L. Wang, Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 37, 136–157 (2017)
H.F. Wang, L. Chen, H. Pang, S. Kaskel, Q. Xu, MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 49, 1414–1448 (2020)
S. Zhao, C. Tan, C.-T. He, P. An, F. Xie, S. Jiang, Y. Zhu, K.-H. Wu, B. Zhang, H. Li, J. Zhang, Y. Chen, S. Liu, J. Dong, Z. Tang, Structural transformation of highly active metal–organic framework electrocatalysts during the oxygen evolution reaction. Nat. Energy 5, 881–890 (2020)
R. Zhang, L. Pan, B. Guo, Z.F. Huang, Z. Chen, L. Wang, X. Zhang, Z. Guo, W. Xu, K.P. Loh, J.J. Zou, Tracking the role of defect types in Co(3)O(4) structural evolution and active motifs during oxygen evolution reaction. J. Am. Chem. Soc. 145, 2271–2281 (2023)
J. He, Y. Liu, Y. Huang, H. Li, Y. Zou, C.L. Dong, S. Wang, Fe2+-induced in situ intercalation and cation exsolution of Co80Fe20(OH)(OCH3) with rich vacancies for boosting oxygen evolution reaction. Adv. Funct. Mater. 31, 2009245 (2021)
Z. Xue, K. Liu, Q. Liu, Y. Li, M. Li, C.Y. Su, N. Ogiwara, H. Kobayashi, H. Kitagawa, M. Liu, G. Li, Missing-linker metal-organic frameworks for oxygen evolution reaction. Nat. Commun. 10, 5048 (2019)
L. Wang, S. Lu, Y. Zhou, X. Guo, Y. Lu, J. He, D.G. Evans, Facile synthesis of metal-organic cobalt hydroxide nanorods exhibiting a reversible structural transition. Chem. Commun. (Camb.) 47, 11002–11004 (2011)
F.T.E. Molis, J.Y. Bottero, O. Barre`s, A. Masion, Chemical and structural transformation of aggregated Al13 polycations, promoted by salicylate ligand. Langmuir 12, 3195-3200 (1995)
D.M.F.Z. Wang, C.C. Ainsworth, S.L. Hemmer, A.G. Joly, M.R. Beversluis, Ground-state proton transfer tautomer of Al(III)-salicylate complexes in ethanol solution. J. Phys. Chem. A 105, 942- 950 (2000)
A.J. Daizy Philip, C. Yohannan Panicker, Hema Tresa Varghese, FT-Raman, FT-IR and surface enhanced Raman scattering spectra of sodium salicylate. Spectrochim. Acta Part A 57, 1561-1566 (2001)
Y. Liu, G. Chen, R. Ge, K. Pei, C. Song, W. Li, Y. Chen, Y. Zhang, L. Feng, R. Che, Construction of CoNiFe trimetallic carbonate hydroxide hierarchical hollow microflowers with oxygen vacancies for electrocatalytic water oxidation. Adv. Funct. Mater. 32, 2200726 (2022)
R.M. Zhaoping Liu, Minoru Osada, Kazunori Takada, Takayoshi Sasaki, Selective and controlled synthesis of α- and β-cobalt hydroxides in highly developed hexagonal platelets. J. Am Chem. Soc. 127, 13869–13874 (2005)
F. Yang, K. Sliozberg, I. Sinev, H. Antoni, A. Bahr, K. Ollegott, W. Xia, J. Masa, W. Grunert, B.R. Cuenya, W. Schuhmann, M. Muhler, Synergistic effect of cobalt and iron in layered double hydroxide catalysts for the oxygen evolution reaction. Chemsuschem 10, 156–165 (2017)
S.K. Das, M.K. Bhunia, M. Motin Seikh, S. Dutta, A. Bhaumik, Highly porous Co(II)-salicylate metal-organic framework: synthesis, characterization and magnetic properties. Dalton Trans. 40, 2932–2939 (2011)
B. Cao, C. Luo, J. Lao, H. Chen, R. Qi, H. Lin, H. Peng, Facile Synthesis of 3d Transition-Metal-Doped alpha-Co(OH)(2) Nanomaterials in water-methanol mediated with ammonia for oxygen evolution reaction. ACS Omega 4, 16612–16618 (2019)
X. Li, H. Zhang, Q. Hu, W. Zhou, J. Shao, X. Jiang, C. Feng, H. Yang, C. He, Amorphous NiFe oxide-based nanoreactors for efficient electrocatalytic water oxidation. Angew. Chem. Int. Ed. Engl. 62, e202300478 (2023)
L. Trotochaud, S.L. Young, J.K. Ranney, S.W. Boettcher, Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 136, 6744–6753 (2014)
J. Hou, Z. Tang, K. Wei, Q. Lai, Y. Liang, Surface reconstruction of Ni doped Co–Fe Prussian blue analogues for enhanced oxygen evolution. Catal. Sci. Technol. 11, 1110–1115 (2021)
X. Chen, Q. Wang, Y. Cheng, H. Xing, J. Li, X. Zhu, L. Ma, Y. Li, D. Liu, S-Doping triggers redox reactivities of both iron and lattice oxygen in FeOOH for low-cost and high-performance water oxidation. Adv. Funct. Mater. 32, 2112674 (2022)
D. Zhou, S. Wang, Y. Jia, X. Xiong, H. Yang, S. Liu, J. Tang, J. Zhang, D. Liu, L. Zheng, Y. Kuang, X. Sun, B. Liu, NiFe hydroxide lattice tensile strain: enhancement of adsorption of oxygenated intermediates for efficient water oxidation catalysis. Angew. Chem. Int. Ed. Engl. 58, 736–740 (2019)
Funding
Open access funding provided by Technical University of Denmark The authors thank GreenLab and Villum Foundation for the financial support. Tao Jiang thanks the Chinese Scholarship Council for the financial support of his PhD study.
Author information
Authors and Affiliations
Contributions
T.J.: investigation, methodology, data curation, and writing original draft. Y.C.Y.: investigation, methodology, and writing original draft. F.Y.W.: visualization, writing, and review. I.A.: visualization, writing, and review. W.J.Z.: supervision, writing, review, and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, T., Yao, Y., Wu, F. et al. Fe-Incorporated Metal-Organic Cobalt Hydroxide Toward Efficient Oxygen Evolution Reaction. Electrocatalysis 15, 273–280 (2024). https://doi.org/10.1007/s12678-024-00871-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-024-00871-0