Abstract
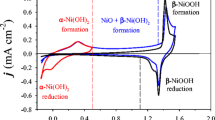
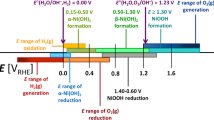
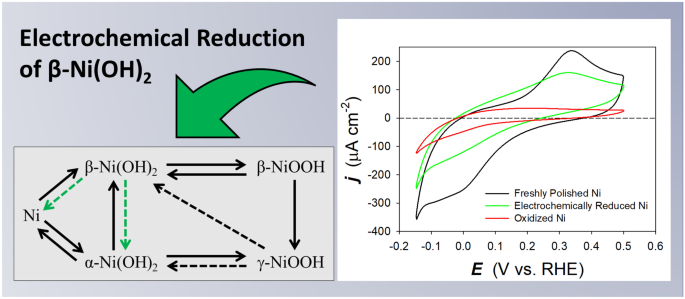
Nickel (Ni) and its (oxy)hydroxide species are an important class of materials that attract significant attention from the scientific community due to their application in alkaline electrochemical energy technologies. Despite their increasing importance, Ni electrochemistry and electrocatalysis are not very well understood. In this article, we contribute to the understanding of the interfacial electrochemical phenomena occurring at polycrystalline Ni electrodes by conducting the first ever systematic analysis of the electrochemical reduction of β-Ni(OH)2. The procedure for the reduction of surface β-Ni(OH)2 is as follows. First, a cyclic voltammetry (CV) measurement is performed in the potential region of β-Ni(OH)2 formation to grow a reproducible hydroxide layer, followed by a linear sweep voltammetry (LSV) measurement conducted between an upper limit potential of Eu = 0.00 V and a lower limit potential (El) ranging between −0.15 V and −0.60 V. The LSV measurement is carried out using very low potential scan rates (s = 0.10, 0.20, 0.50, and 1.0 mV s−1). The analysis of the electrochemical reducibility of the surface β-Ni(OH)2 layer is accomplished by comparing the CV transient of a freshly polished Ni electrode and the CV transient recorded after the LSV experiment that is designed to reduce the β-Ni(OH)2 layer. The influence of different experimental parameters of the above-described procedure is analyzed independently, namely, (i) the lower potential limit of the LSV measurement, (ii) the potential scan rate of the LSV measurement, (iii) the direction of the LSV measurement (anodic or cathodic), (iv) the thickness of the surface layer of β-Ni(OH)2, and (v) the cathodic polarization of the electrode for different polarization times. Our findings demonstrate that, contrary to an existing belief, the reduction of β-Ni(OH)2 can be achieved electrochemically. Features characteristic of the formation and reduction of α-Ni(OH)2 are observed in the CV transients acquired after employing the procedure for the electrochemical reduction of β-Ni(OH)2, indicative that a metallic Ni surface is regenerated. The analysis of the charge density value of the anodic peak in the CV transient also points to the electrochemical reduction of β-Ni(OH)2 to metallic Ni. These findings lead to an updated version of the Bode diagram and might have significant implications for alkaline electrochemical energy technologies using Ni-based materials.
Graphical abstract










Similar content being viewed by others
References
P. Oliva, J. Leonardi, J.F. Laurent, C. Delmas, J.J. Braconnier, M. Figlarz, F. Fievet, A. de Guibert, Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J. Power Sources 8, 229 (1982)
D.S. Hall, D.J. Lockwood, C. Bock, and B.R. MacDougall, Nickel hydroxides and related materials: a review of their structures, synthesis and properties. Proc. R. Soc. A Math. Phys. Eng. Sci. 471, 20140792 (2015).
J. McBreen, Handbook of Battery Materials, 2nd edn. (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011).
D. Linden, Nickel-Metal Hydride Batteries, 4th edn. (McGraw-Hill, New York, 2011).
E. Gülzow, Alkaline fuel cells: a critical review. J. Power Sources 61, 99 (1996)
M. Schalenbach, G. Tjarks, M. Carmo, W. Lueke, M. Mueller, D. Stolten, Acidic or alkaline? Towards a new perspective on the efficiency of water electrolysis. J. Electrochem. Soc. 163, F3197 (2016)
M. Bodner, A. Hofer, V. Hacker, H2 generation from alkaline electrolyzer. Wiley Interdiscip. Rev. Energy Environ. 4, 365 (2015)
G. McLean, An assessment of alkaline fuel cell technology. Int. J. Hydrogen Energy 27, 507 (2002)
J. Van Drunen, B. Kinkead, M.C.P. Wang, E. Sourty, B.D. Gates, G. Jerkiewicz, Comprehensive structural, surface-chemical and electrochemical characterization of nickel-based metallic foams. ACS Appl. Mater. Interfaces 5, 6712 (2013)
V. Costa Bassetto, M. Mensi, E. Oveisi, H.H. Girault, and A. Lesch, Print-light-synthesis of Ni and NiFe- nanoscale catalysts for oxygen evolution. ACS Appl. Energy Mater. 2, 6322 (2019).
S. Kabir, K. Lemire, K. Artyushkova, A. Roy, M. Odgaard, D. Schlueter, A. Oshchepkov, A. Bonnefont, E. Savinova, D.C. Sabarirajan, P. Mandal, E.J. Crumlin, I.V. Zenyuk, P. Atanassov, A. Serov, Platinum group metal-free NiMo hydrogen oxidation catalysts: high performance and durability in alkaline exchange membrane fuel cells. J. Mater. Chem. A 5, 24433 (2017)
K.M. Cole, S. Tahmasebi, S. Baranton, G. Jerkiewicz, D.W. Kirk, S.J. Thorpe, Erratum: In Situ Raman Study of Amorphous and Crystalline Ni-Co Alloys for the Alkaline Oxygen Evolution Reaction. J. Electrochem. Soc., 165, J3122 (2018), J. Electrochem. Soc. 166, X1 (2019)
S. Fujita, I. Nagashima, Y. Sunada, Y. Nishiki, S. Mitsushima, Electrocatalytic activity and durability of LixNi2-xO2/Ni electrode prepared by oxidation with LiOH melt for alkaline water electrolysis. Electrocatalysis 8, 422 (2017)
O.V. Cherstiouk, P.A. Simonov, A.G. Oshchepkov, V.I. Zaikovskii, T.Y. Kardash, A. Bonnefont, V.N. Parmon, E.R. Savinova, Electrocatalysis of the hydrogen oxidation reaction on carbon-supported bimetallic NiCu particles prepared by an improved wet chemical synthesis. J. Electroanal. Chem. 783, 146 (2016)
E.S. Davydova, J. Zaffran, K. Dhaka, M.C. Toroker, D.R. Dekel, Hydrogen oxidation on Ni-based electrocatalysts: The effect of metal doping. Catalysts 8, 1 (2018)
E.S. Davydova, F.D. Speck, M.T.Y. Paul, D.R. Dekel, S. Cherevko, Stability limits of Ni-based hydrogen oxidation electrocatalysts for anion exchange membrane fuel cells. ACS Catal. 9, 6837 (2019)
S. Fujita, I. Nagashima, Y. Nishiki, C. Canaff, T.W. Napporn, S. Mitsushima, The effect of LixNi2-xO2/Ni with modification method on activity and durability of alkaline water electrolysis anode. Electrocatalysis 9, 162 (2018)
Y. Uchino, T. Kobayashi, S. Hasegawa, I. Nagashima, Y. Sunada, A. Manabe, Y. Nishiki, S. Mitsushima, Dependence of the reverse current on the surface of electrode placed on a bipolar plate in an alkaline water electrolyzer. Electrochemistry 86, 138 (2018)
Y. Uchino, T. Kobayashi, S. Hasegawa, I. Nagashima, Y. Sunada, A. Manabe, Y. Nishiki, S. Mitsushima, Relationship between the redox reactions on a bipolar plate and reverse current after alkaline water electrolysis. Electrocatalysis 9, 67 (2018)
A.G. Oshchepkov, A. Bonnefont, V.N. Parmon, E.R. Savinova, On the effect of temperature and surface oxidation on the kinetics of hydrogen electrode reactions on nickel in alkaline media. Electrochim. Acta 269, 111 (2018)
D.S. Hall, C. Bock, B.R. MacDougall, The electrochemistry of metallic nickel: oxides, hydroxides, hydrides and alkaline hydrogen evolution. J. Electrochem. Soc. 160, F235 (2013)
H. Bode, K. Dehmelt, and J. Witte, Zur kenntnis der nickelhydroxidelektrode—I.Über das nickel (II)-hydroxidhydrat. Electrochim. Acta 11, 1079 (1966).
M. Alsabet, M. Grden, and G. Jerkiewicz, Electrochemical growth of surface oxides on nickel. Part 2: formation of β-Ni(OH)2 and NiO in relation to the polarization potential, polarization time, and temperature. Electrocatalysis 5, 136 (2014).
B. Beverskog, I. Puigdomenech, Revised Pourbaix diagrams for nickel at 25–300 °C. Corros. Sci. 39, 969 (1997)
S.G. Bratsch, Standard electrode potentials and temperature coefficients in water at 298.15 K. J. Phys. Chem. Ref. Data 18, 1 (1989).
N. Arulmozhi, D. Esau, J. van Drunen, G. Jerkiewicz, Design and development of instrumentations for the preparation of platinum single crystals for electrochemistry and electrocatalysis research part 3: final treatment, electrochemical measurements, and recommended laboratory practices. Electrocatalysis 9, 113 (2018)
G. Jerkiewicz, Standard and reversible hydrogen electrodes: theory, design, operation, and applications. ACS Catal. 10, 8409 (2020)
S.A.S. Machado, L.A. Avaca, Hydrogen evolution reaction on nickel surfaces stabilized by H-absorption. Electrochim. Acta 39, 1385 (1994)
M. Alsabet, M. Grden, and G. Jerkiewicz, Electrochemical growth of surface oxides on nickel. Part 1: formation of α-Ni(OH)2 in relation to the polarization potential, polarization time, and temperature. Electrocatalysis 2, 317 (2011).
M. Grdeń, M. Alsabet, G. Jerkiewicz, Surface science and electrochemical analysis of nickel foams. ACS Appl. Mater. Interfaces 4, 3012 (2012)
G. Jerkiewicz, Hydrogen sorption at/in electrodes. Prog. Surf. Sci. 57, 137 (1998)
E.B. Ferreira, S. Tahmasebi, G. Jerkiewicz, On the catalytic activity and corrosion behavior of polycrystalline nickel in alkaline media in the presence of neutral and reactive gases. Electrocatalysis (2020). https://doi.org/10.1007/s12678-020-00637-4
S. Tahmasebi, M.A. Hossain, G. Jerkiewicz, Corrosion behavior of platinum in aqueous H2SO4 solution: part 1—influence of the potential scan rate and the dissolved gas. Electrocatalysis 9, 172 (2018)
M. Alsabet, M. Grden, and G. Jerkiewicz, Electrochemical growth of surface oxides on nickel. Part 3: formation of β-NiOOH in relation to the polarization potential, polarization time, and temperature. Electrocatalysis 6, 60 (2014).
G. Jerkiewicz, G. Vatankhah, J. Lessard, M.P. Soriaga, Y.-S. Park, Surface-oxide growth at platinum electrodes in aqueous H2SO4. Electrochim. Acta 49, 1451 (2004)
Acknowledgements
This research was conducted as part of the Engineered Nickel Catalysts for Electrochemical Clean Energy project administered from Queen’s University and supported by grant number RGPNM 477963-2015 under the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Frontiers Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira, E.B., Jerkiewicz, G. On the Electrochemical Reduction of β-Ni(OH)2 to Metallic Nickel. Electrocatalysis 12, 199–209 (2021). https://doi.org/10.1007/s12678-021-00643-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-021-00643-0