Abstract
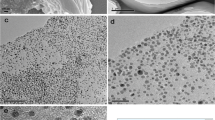
Polydopamine functionalized reduced graphene oxide-silver nanoparticle (PDA-RGO/Ag NP) nanocomposites were successfully prepared by a simple and mild procedure. Graphene oxide (GO) sheets were firstly coated with PDA via a self-polymerization process which provided an excellent interface for in-situ growing silver nanoparticles. Fourier transform infrared spectroscopy (FTIR) confirmed the successful coating of PDA and informed the reduction of the surface functional groups of GO. The formation of reduced GO and silver NPs was further evidenced by UV-Vis and X-ray diffraction spectroscopy. The as-prepared PDA-RGO/Ag nanocomposites could greatly enhance the electrochemical reduction of hydrogen peroxide (H2O2). This excellent performance was attributed to the increased effective electrode surface area due to the deposition of nano-sized Ag particles and graphene. The PDA-RGO/Ag-based electrochemical sensor displayed a rapid amperometric response for H2O2 measurement with a wide linear range from 0.5 μM to 8 mM and a low detection limit of 2.07 μM.





Similar content being viewed by others
References
M. Abo, Y. Urano, K. Hanaoka, T. Terai, T. Komatsu, T. Nagano, Development of a highly sensitive fluorescence probe for hydrogen peroxide. J. Am. Chem. Soc. 133, 10629 (2011)
T. Jiao, B.D. Leca-Bouvier, P. Boullanger, L.J. Blum, A.P. Girard-Egrot, Electrochemiluminescent detection of hydrogen peroxide using amphiphilic luminol derivatives in solution. Colloids Surf. A. 321, 143 (2008)
A.C. Pappas, C.D. Stalikas, Y.C. Fiamegos, M.I. Karayannis, Determination of hydrogen peroxide by using a flow injection system with immobilized peroxidase and long pathlength capillary spectrophotometry. Anal. Chim. Acta 455, 305 (2002)
A. Yu, Q. Wang, J. Yong, P.J. Mahon, F. Malherbe, F. Wang, Silver nanoparticle–carbon nanotube hybrid films: preparation and electrochemical sensing. Electrochim. Acta 74, 111 (2012)
D. Lu, Y. Zhang, S. Lin, L. Wang, C. Wang, Synthesis of PtAu bimetallic nanoparticles on graphene–carbon nanotube hybrid nanomaterials for nonenzymatic hydrogen peroxide sensor. Talanta 112, 111 (2013)
J.H. Yang, K. Zhang, D. Zhang, Gourd-shaped silver nanoparticle–graphene composite for electrochemical oxidation of glucose. Mater. Lett. 97, 133 (2013)
A. Yu, Carbon nanotubepolypyrrole hybrid films as potentiometric peroxide biosensors. Chem. Lett. 41(1492) (2012)
G.A. Moradi, N.M. Huang, H.N. Lim, R. Zakaria, C.Y. Yin, One-step electrodeposition synthesis of silver-nanoparticle-decorated graphene on indium-tin-oxide for enzymeless hydrogen peroxide detection. Carbon 62(405) (2013)
M.R. Guascito, D. Chirizzi, R.A. Picca, E. Mazzotta, C. Malitesta, Ag nanoparticles capped by a nontoxic polymer: electrochemical and spectroscopic characterization of a novel nanomaterial for glucose detection. Mater. Sci. Eng., C 31, 606 (2011)
Z. Zhang, J. Zhang, B. Zhang, J. Tang, Mussel-inspired functionalization of graphene for synthesizing Ag-polydopamine-graphene nanosheets as antibacterial materials. Nanoscale 5, 118 (2013)
H. Lee, S.M. Dellatore, W.M. Miller, P.B. Messersmith, Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426 (2007)
L. Zheng, L. Xiong, Y. Li, J. Xu, X. Kang, Z. Zou, Facile preparation of polydopamine-reduced graphene oxide nanocomposite and its electrochemical application in simultaneous determination of hydroquinone and catechol. Sens. Actuators, B 177, 344 (2013)
R.A. Zangmeister, T.A. Morris, M.J. Tarlov, Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 29, 8619 (2013)
Y. Zhou, Q. Bao, L.A.L. Tang, Y. Zhong, P.K. Loh, Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem. Mater. 21, 2950 (2009)
M.Y. Wang, T. Shen, M. Wang, D. Zhang, J. Chen, One-pot green synthesis of Ag nanoparticles-decorated reduced graphene oxide for efficient nonenzymatic H2O2 biosensor. Mater. Lett. 107, 311 (2013)
A. Bello, M. Fabiane, A.D. Dodoo, K.I. Ozoemena, N. Manyala, Silver nanoparticles decorated on a three-dimensional graphene scaffold for electrochemical applications. J. Phys. Chem. Solid 75, 109 (2014)
K.J. Huang, L. Wang, J. Li, M. Yu, Y.M. Liu, Electrochemical sensing of catechol using a glassy carbon electrode modified with a composite made from silver nanoparticles, polydopamine, and graphene. Microchim. Acta 180, 751 (2013)
Acknowledgments
L Fu acknowledges the Swinburne University Postgraduate Research Award (SUPRA) for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, L., Lai, G., Jia, B. et al. Preparation and Electrocatalytic Properties of Polydopamine Functionalized Reduced Graphene Oxide-Silver Nanocomposites. Electrocatalysis 6, 72–76 (2015). https://doi.org/10.1007/s12678-014-0219-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-014-0219-9