Abstract
In the quest for early cancer diagnosis, early identification and treatment are paramount. Recently, ctDNA detection has emerged as a viable avenue for early screening of cancer. The examination of ctDNA in fluid biopsies has gained substantial attention in tumor diagnosis and therapy. Both the scientific community and industry are actively exploring this field. However, developing cost-effective, portable, and real-time ctDNA measurement methods using conventional gene detection equipment poses a significant challenge. This challenge has led to the exploration of alternative approaches. Electrochemical biosensors, distinguished by their heightened sensitivity, remarkable specificity, affordability, and excellent portability, have emerged as a promising avenue for ctDNA detection. This review is dedicated to the specific focus on ctDNA detection, highlighting recent advancements in this evolving detection technology. We aimed to reference previous studies related to ctDNA-targeted cancer detection using electrochemical biosensors to advocate the utilization of electrochemical biosensors in healthcare diagnostics. Further research is imperative for the effective integration of ctDNA analysis into point-of-care cancer testing. Innovative approaches utilizing multiple markers need to be explored to advance this technology and make substantial contributions to societal well-being.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Worldwide, cancer is the leading cause of death and the primary limiting factor of life expectancy [1]. An aura of belief for cancer patients was created due to the introduction of precision medicine in the field of medical oncology, which has significantly improved over the past several decades as a result of the swift expansion of knowledge of cancer genomics and the discovery of suitable genomic biomarkers [2]. Although the identification of therapeutic biomarkers is a revolutionary development in the management of cancer patients, it has also led to a number of difficulties. For instance, the availability of tumor tissue in patients with lung cancer is compromised by the growing number of biomarkers to be evaluated. Furthermore, tissue biopsy, in addition to being an extremely invasive process with possible risks for patients [3], does not reflect tumor heterogeneity [4], thus rendering it more difficult to provide an overview of the molecular properties of the tumor.
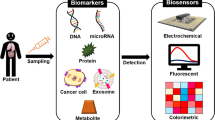
In this case, liquid biopsy appeared to be a minimally invasive method that allows the detection of important tumor-derived biomarkers across the course of the disease, including resistance mutations [5, 6]. Tumor-educated platelets (TEPs), circulating tumor cells (CTCs), extracellular vesicles (EVs), and ctDNA are among the components that can be separated from bodily fluids and utilized in liquid biopsy analysis [7]. Among them, we will concentrate on ctDNA because it has the highest diagnostic and prognostic potential. ctDNA comprises single- or double-stranded DNA molecules released into the bloodstream due to the death and degradation of circulating tumor cells [8]. It has great potential as a diagnostic biomarker for early-stage cancer. Previous research has shown that ctDNA encompasses many cancer-related molecular characteristics, such as point mutations, methylation and cancer-derived viral sequences. ctDNA is usually present in the blood stream in the form of single- and double-stranded DNA entities from multiple origins. ctDNA serves as an efficient non-invasive diagnostic tool in personalized treatments [9].
The identification of ctDNA is an important step in its clinical application. Polymerase chain reaction (PCR) [10] and DNA sequencing [11] are two conventional DNA detection methods that are used to identify ctDNA. The majority of traditional methods for detecting ctDNA involve lengthy testing times, laborious procedures, expensive equipment, and inconvenient handling [12]. These factors have led to some limitations in the use of ctDNA in clinical settings. Therefore, the development of a low-cost, real-time, portable, and convenient device to detect ctDNA is imperative for cancer screening and diagnosis. The rapid development of biosensing technology has led to a new approach for the detection of ctDNA. In contrast to existing detection methods, ctDNA biosensors exhibit advantages in terms of cost-effectiveness, heightened sensitivity, and simplified integration, enhancing their suitability for on-site detection and portable healthcare investigations [13]. The widespread use of electrochemical sensors is attributed to their simplicity and the ease of miniaturization, which makes them highly versatile. Furthermore, these sensors offer rapid analysis and exceptional sensitivity and are relatively cost-effective [14]. As a result, electrochemical transducers are well suited for the development of on-site detection devices.
2 Circulating tumor DNA (ctDNA)
ctDNA refers to ssDNA or dsDNA fragments released from neoplastic cells, normally comprising 1% or less of the total cell-free DNA (cfDNA) [15]. Ground breaking research revealed that heightened cells can turn into a cancerous tumors and lead to an elevated concentration of cfDNA in the bloodstream of cancer patients, a finding later corroborated by other researchers [16]. It is important to note, however, that various conditions not associated with cancer, including ischemia, acute trauma, infection, or inflammation, can also increase the amount of cell-free DNA in circulation. ctDNA is primarily discharged into the bloodstream through processes such as apoptosis, necrosis, and phagocytosis and is often transported by exosomes. Furthermore, ctDNA is identifiable not only in blood but also in other bodily fluids, including urine, sputum, saliva, stool, pleural fluid, and cerebrospinal fluid (CSF) [17]. Neoplastic cells consistently release these DNA fragments, which are quickly broken down by blood nucleases and then eliminated by the kidneys and liver [18]. This process contributes to their brief half-life in circulation, which lasts from 16 to 150 min. The swift turnover of ctDNA in the bloodstream positions it as a compelling target for attaining instantaneous insights into mutation dynamics and tumor burden (Fig. 1). Moreover, DNA fragments originating from malignant cells often exhibit shorter lengths, prompting the selection of fragments within the range of 90 to 150 base pairs to increase the sensitivity of ctDNA assays [19]. It is essential to highlight that plasma samples are favored over serum samples for ctDNA analysis. This preference arises because serum samples have higher background-to-signal ratios because they contain more DNA from leukocytes that have been lysed during the clotting process and cause interference with the assays.
2.1 Conventional ctDNA detection methods
2.1.1 PCR-Based technologies
Traditional PCR-based technologies are easy to use and inexpensive. However, most of these methodologies have several limitations, such as low sensitivity, limited optimization and the ability to detect only previously known genes and mutations. Various PCR-based protocols have been reported over the years, including digital PCR (dPCR) and BEAMing (beads, emulsification, amplification, and magnetics) for ctDNA detection. Digital PCR (dPCR) has gained popularity for ctDNA diagnosis due to its increased sensitivity and user-friendly characteristics [20]. It is a robust technique that allows the precise and accurate quantification of target molecules through sample dilution and partitioning into multiple compartments. On the other hand, BEAMing is based on emulsion PCR, which includes DNA amplification on beads. BEAM is useful for accurately screening template DNA diversity by determining the fraction of particular mutations in the DNA population [21]. These techniques excel in the precise detection of point mutations, even at frequencies as low as ≤ 0.1%. However, these methods are restricted by their ability to analyze only a limited number of genomic loci and the necessity of prior knowledge about the investigated genomic aberrations [22]. In the last decade, there has been significant momentum in research focused on the early diagnosis of cancer using ctDNA (Table 1).
3 NGS-Based technologies
As ctDNA only comprises a portion of cfDNA, it is critical to use cancer-specific modifications to differentiate polymorphisms between ctDNA and its wild-type counterpart. The majority of current approaches that use ctDNA as a cancer biomarker focus on mutation detection [42]. NGS-based approaches cover a wide variety of mutations by analyzing the entire sequences of relevant genes. NGS techniques, whether targeted or untargeted, can be applied to ctDNA analysis [43]. Targeted methods for ctDNA profiling typically involve sequencing a large number of genes that can target hundreds of genes within the entire exome. With the relatively low throughput and high sensitivity of NGS techniques, NGS can be used to identify sequence-specific regions of genes of interest that cover clinically significant mutations. Targets are enriched by applying hybridization capture techniques or multiplex PCR to confirm the maximum amplification of target gene fragments. Owing to improved sensitivity and specificity, targeted sequencing of mutated genes is an option for medical diagnosis. In untargeted techniques, the entire genome is sequenced without performing an enrichment step. While whole-genome sequencing increases the depth of sequencing, it is suitable for basic biomedical research because it aids in identifying novel genomic abnormalities associated with early disease diagnosis or therapeutic strategies [44].
In the case of ctDNA profiling, factors such as cost, accuracy and speed are pivotal considerations, especially in diagnostic scenarios. In this context, various platforms are better suited for ctDNA sequencing, with Illumina sequencing by synthesis (SBS) being one of the prominent options, in addition to Ion Torrent and nanopore sequencing. The Illumina sequencing platform remains dominant due to its high throughput and precision. In contrast, the Ion Torrent semiconductor-based sequencer boasts a faster run speed despite lower throughput, which proves advantageous in situations where speed is necessary. In recent years, the accuracy of nanopore sequencing has improved; nevertheless, nanopore sequencing remains behind other mainstream sequencing technologies [45]. Nevertheless, it offers advantages in terms of its easy sequencing and library preparation. Hence, in situations where poor accuracy is not necessary, such as copy number variation profiling, nanopore sequencing can be successfully used for ctDNA analysis [46].
Using appropriate bioinformatics algorithms is also essential for mutation detection after the creation of raw sequencing data. Additionally, there are databases available to aid in interpreting liquid biopsy data. One such database, liqDB, focuses on short RNA sequencing profiles from liquid biopsies. It offers users valuable insights for navigating small RNA liquid biopsy research, addressing both technical challenges and theoretical hurdles [47]. ctcRbase serves as a database for accessing and exploring gene tools and is applicable for querying and browsing gene expression data from circulating tumor cells/micro emboli. The critical aspect of accurately identifying ctDNA amidst a significant background of cfDNA lies in effectively detecting alterations specific to ctDNA compared to normal cfDNA. Among these alterations, single nucleotide mutations are most frequently observed. Specifically, in the context of ctDNA sequencing, the sensitivity of detecting the mutant allele frequency (MAF) or variant allele frequency (VAF) is commonly used to evaluate the effectiveness of NGS in a ctDNA profiling assay. The MAF or VAF represents the proportion of DNA molecules carrying a mutation compared to the total number of molecules containing the same allele. Therefore, these findings indicate that the presence of ctDNA within cfDNAs contains tumor-specific mutant alleles. Therefore, the ability to detect a lower MAF signifies more sensitivity in NGS assays for ctDNA analysis [48]. Therefore, numerous tools are available for analyzing small RNA high-throughput sequencing data downstream, covering tasks such as predicting, screening, identifying, and confirming miRNA targets. Among these tools are miRDeep, ShortStack, sRNAbench, SeqBuster, and miRTrace, each contributing to the advancement of quality control and the study of ctDNA [49].
4 Hybrid techniques
Hybrid techniques such as the combination of PCR and NGS avoid the limitations of both methods, such as the sensitivity of NGS (1–2%) and the previously required knowledge of the target in the case of PCR. Clark developed the Foundation ACT assay, a next-generation sequencing technique using hybrid capture that allows for the genomic profiling of ctDNA derived from blood [50]. This assay, characterized by high sequencing coverage and molecular barcode-based error detection, excels in detecting genomic alterations, including short variants, genomic rearrangements, and copy number amplifications at low allele frequencies. Analytical validation on 2666 reference alterations demonstrated impressive sensitivity, surpassing 99% for short variants at an AF > 0.5%. Genomic alteration frequencies between ctDNA and tissue are revealed by the clinical application of Foundation ACT, highlighting its usefulness in non-invasive genomic profiling with strong agreement with tissue-based assays.
In the realm of cancer diagnostics, the examination of circulating cell-free DNA (cfDNA) extracted from the blood of cancer patients has emerged as a non-invasive strategy for scrutinizing somatic tumor alterations. In a study, the Memorial Sloan Kettering - Analysis of Circulating cfDNA to Examine Somatic Status (MSK-ACCESS), a hybridization capture-based next-generation sequencing (NGS) assay tailored for the detection of remarkably low-frequency somatic alterations across 129 genes, was used [50]. Investigative validation underscores the robustness of the assay, achieving 92% sensitivity for non-inheritable mutations calling down to 0.5% allele frequency and 99% for anticipated mutation profiling. Notably, somatic alterations were identified in approximately 73% of the samples, with 56% of them harboring clinically actionable alterations. A distinctive aspect of MSK-ACCESS is its incorporation of matched normal sequencing, enabling the exclusion of more than 10,000 germ line and clonal hematopoietic variants while maintaining relevant somatic variations. This comprehensive approach underscores the significance of examining paired normal samples when interpreting cfDNA findings, emphasizing the pivotal role of cfDNA as a genomic profiling tool for cancer patients.
Klega and peers addressed the prevalence of detectable ctDNA in pediatric patients with poorly understood tumors [51]. NGS was used to identify and quantify ctDNA in the blood of pediatric cancer patients with prevalent solid tumors. In pediatric solid tumors, structural variants are more frequent than recurrent point mutations. An ultralow passage whole-genome sequencing method was adapted to record variations in copy numbers, coupled with an assay for hybrid capture sequencing for translocation detection in pediatric patient’s liquid biopsy samples. The results revealed that whole-genome sequencing using ultralow passage DNA identified ctDNA in patients with osteosarcoma alveolar rhabdomyosarcoma, neuroblastoma, and Wilms tumor. For Ewing sarcoma, detecting EWSR1 alterations is a more sensitive approach. Recently, in 2023, Zhao J et al. studied the effectiveness of a personalized cancer monitoring assay for ctDNA detection from malignant tumors using whole exome sequencing [52]. While ctDNA detection features were assessed through analytical validation involving nine commercial reference samples and 250 human specimens, this validation resulted in 1349 exome-sequenced libraries derived from cell-free DNA. Overall, whole-exome sequencing exhibited a specificity of 99.9% and sensitivity greater than 99.8%. Patient-specific panels were effectively devised for all samples with a tumor content of at least 20%. The ctDNA detection component demonstrated a specificity surpassing 99.9% and a sensitivity of 96.3%, demonstrating the ability of the assay to detect ctDNA with high accuracy across different conditions and thresholds.
5 Electrochemical biosensors
In the realm of biosensors, electrochemical sensors stand out as a predominant choice for analytical purposes. Renowned for its ability to detect biomaterials based on electrochemical properties influenced by the attributes of the intended material, this sensor type utilizes the target material’s selective reactivity with the bio-receptor [53]. It often employs nucleic acid interactions or antigen-antibody reactions to enhance sensitivity. The advantages of electrochemical sensors include a straightforward analysis method, outstanding performance at low cost of production [54], and significant miniaturization capabilities [55]. These sensors are generally categorized into impedance sensors, current sensors, and voltage sensors based on the transducer used [56, 57]. Impedance-based sensors, a subset of electrochemical sensors, facilitate fast and sensitive analysis by measuring electrical properties that impede current flow [58]. However, challenges such as reduced power and current flow obstruction have prompted strategies involving labeling molecules such as DNA, enzymes, nanoparticles, and carbon materials. On the other hand, current- and voltage-based sensors measure oxidation‒reduction signals, offering high sensitivity, albeit limited to substances with electrochemical activity [59]. Leveraging nanotechnology and advanced fabrication methods, there is a growing trend towards developing compact and robust sensing systems suitable for on-site analyses. Screen-printing equipment, which has existed for centuries, has gained prominence in the last two decades, particularly in biosensor applications. Nanotechnology has enabled the fabrication of printed devices on flexible substrates, leading to the creation of highly reproducible, disposable, stable, and affordable screen-printed electrodes (SPEs), significantly influencing electrochemical biosensor development. SPEs, with their easily modifiable ink, offer adaptability, accuracy, and versatility, particularly in reference, working, and counter electrodes.
Moving into the core components of electrochemical biosensors, three essential elements play a vital role. The first involves biomolecular recognition components, such as organelles, nucleic acids, enzymes, and immunological compounds, known for their specificity in discerning the intended analyte. Techniques such as physical adsorption, covalent attachment, encapsulation, and self-assembled monolayers are employed to immobilize these components onto the biosensor surface [60]. The second component comprises conductive electrodes, which are crucial for signal conversion and are made from materials such as graphene, carbon, or gold. They facilitate the conversion of biological interactions into electrical signals, aiding in the quantification of target analyte concentrations. The third component is a signal transduction system based on the principles of electrochemistry, allowing the determination of substance concentration and composition through their electrochemical properties. In the context of biosensors, the combination of biomolecular recognition, conductive electrodes, and signal transduction mechanisms forms the basis for their efficacy. One notable application of electrochemical biosensors is in the detection of ctDNA. These sensors prove highly capable of efficiently detecting ctDNA, given the wide range of surface alteration techniques and the application of diverse transducers. While most clinical evaluations of ctDNA involve methods such as gene sequencing and PCR [61], ctDNA-based biosensors offer increased sensitivity, ease of integration, and cost-effectiveness for on-site detection and portable healthcare investigations [62].
5.1 Electrochemical biosensor and its techniques
Electrochemical biosensor comprises a utilization of electro analytical techniques on biological components. Biological components in biosensor interact to single or multiple analyte, resulting into a fluctuation in electrical signals monitored by transducers. Sensor is a part of electrochemical cell that usually consists of three electrodes, reference electrode typically made of silver chloride, a working electrode formed of chemically stable conductive material viz., platinum, gold and carbon while the third one is auxiliary electrode. Electrochemical techniques are generally categorized into three main types of measurements: current, potential, and impedance.
6 Amperometric/voltammetric techniques
Amperometric/voltammetry technique is a simple technique which usually referred for bio-catalytic and affinity sensor. it is an electroanalytical technique that derives information about one or more analyte by measuring the current potential (reduction or oxidation) as a function of the applied potential [60]. The analyte involved in the reaction are measured using the current. Voltammetric technique works in principle of detection at fixed potential when there is a negligible change in the applied current. Current here is directly correlated to the bulk concentration of electroactive species or its production or degradation rate within the adjacent bio-catalytic layer. Study state of current is due to the direct dependency of bio-catalytic reaction rate over analyte concentration. Amperometric transducer measures the charge transfers between two electrodes in the presence of electrolyte. Biosensors can employ a verity of voltammetric techniques, including cyclic voltammetry (CV), differential pulse voltammetry (DPV) and square wave voltammetry (SWV) [63]. While in amperometric techniques, generated current change due to oxidation and reduction are recorded over a time while the potential of working electrode remains constant. Amperometric sensors were considered as a subclass of the voltammetric sensors and major distinguishing factor between amperometry and voltammetry is the absence of scanning potential [64]. Current was measured when desired potential value is stepped and holding the potential for uniform current flow across electrode [65].
7 Potentiometric techniques
This technique is based on measurement of accumulated charge at working electrode with respect to reference electrode when there is negligible or non-significant flow of current [66]. Several type of experiments such as cyclic voltammetry, square wave voltammetry, and stripping voltammetry. This method does not deplete the species being tested, as we observe in the case of amperometric biosensors. By comparing its performance to a reference electrode, it is possible to expect a linear relationship between the analyte concentration and its response [63].
7.1 Impedimetric techniques
Impedimetric technique is utilized for measuring change in resistance or conductance due to interaction of analyte to biological component. The impendence biosensor is typically an integral part of the Wheatstone bridge, where produced ions can significantly increase impedance. In impedimetric biosensors, electrochemical impedance spectroscopy (EIS) is the most often utilized approach where impedance is recorded over various frequencies of alternating current potential [64]. Physico-chemical changes due to the interaction of analyte to a biological component provide important information of frequency-domain response. EIS also utilized as a characterization technique as it informs the layer-by-layer fabrication of biosensing components leading to change in conductance. During last two decades, application of EIS in electrochemical biosensing platforms has rapidly increased, making it one of the most promising characterization technique [67].
7.2 Electrochemical biosensor-based approach for detecting ctDNA
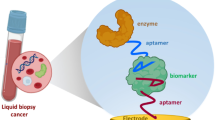
The detection of ctDNA, acknowledged as a groundbreaking technology, has significant potential in cancer diagnostics because it originates from circulating tumor cells during processes such as necrosis and apoptosis [68]. General ctDNA detection techniques include digital PCR, droplet digital PCR, magnetic bead particle amplification, tag amplified depth sequencing, and others. However, these technologies have the limitations of high cost, low mobility, numerous false positives, and long assay times, making them unsuitable for on-site monitoring and portable healthcare. In contrast, ctDNA electrochemical biosensors exhibit high potential for on-site and mobile detection because of their specificity, portability, sensitivity, and rapid response [69]. A ctDNA-based electrochemical biosensor is designed to transform the biotic signal produced by the specific binding of the target ctDNA to the recognition probes into an electrical signal that can be detected (Fig. 2). This biosensor utilizes electrochemical impedance at multiple frequencies to analyze biological information. In the context of health, electrochemical biosensors are valuable tools for personalized medicine because of their reasonable cost, mobility, and real-time detection. These qualities make them an ideal technique for on-site selection and portable health observation [59].
This part of the review focuses on recent advancements in ctDNA electrochemical biosensors, particularly emphasizing the types of recognition elements on electrode surfaces. Conductive electrodes, often made of gold, carbon, or graphene, play a crucial role in signal conversion. The signal transduction mechanism utilizes electrochemical approaches such as cyclic voltammetry, differential pulse voltammetry, square wave voltammetry, and electrochemical impedance spectroscopy to determine substance composition and content based on electrochemical properties [70]. As we delve into the realm of ctDNA biosensors, subsequent sections explore various biosensing approaches customized for ctDNA identification, specifically for cancer detection.
7.3 Nucleic acid probe-based diagnosis
Nucleic acid-based sensors are frequently applied in medical diagnostics, pathogen recognition and environmental monitoring owing to their pronounced sensitivity, rapid response, user friendliness, affordability, and potential for miniaturization. These biosensors employ specific hybridization to detect the target DNA molecule, giving rise to an electric signal amenable to analysis.
7.4 PNA Probe
Peptide nucleic acid (PNA) has become a key component of DNA sensors for the selective interaction and capture of ctDNA. PNA demonstrates enhanced hybridization capabilities with DNA molecules compared to DNA/DNA interactions due to the lack of electrostatic repulsion among these entities [71]. The sulfhydryl group of PNA binds to the surface of electrode effectively by forming an covalent bond (Au-S) with the gold electrode [72]. This immobilization process guarantees the stable attachment of the PNA probe to the substrate of the sensor. This process is accomplished through covalently bond modifications. This method was utilized to measure ctDNA for identifying particular methylation sites in the PIK3CA gene. The ctDNA, comprising various tumor-specific abnormalities, such as DNA mutations and epigenetic alterations, holds immense promise as a real-time biomarker for non-invasive cancer evaluation. Cai et al. announced a novel platform for biomarker detection, employing peptide nucleic acid probe conjugated with gold nanoparticles and lead phosphate Apoferritin [73]. This platform integrates a DNA biosensor capable of quantifying ctDNA by detecting tumor specific methylation and mutations in the PIK3CA gene. By utilizing a PNA probe with an anti-5-methylcytosine monoclonal antibody (anti-5-mC), a multilayer assembly is formed on a screen printed electrode surface. Additionally, gold nanoparticles and lead phosphate apoferritin were presented to facilitate double amplification. The proposed DNA sensor exhibits a lineal current response with ctDNA dilutions ranging from 50 to 10,000 fM and gives a limit of detection of 10 fM. Importantly, it effectively detected ctDNA in the suspected cancer positive blood serums, indicating its potential in diverse malignancy detection and personalized cancer therapy facilitation (Fig. 3) (Table 2).
A DNA clutch probe approach was used to prevent the hybridization of single stranded DNA (ssDNA) segments to increase sensitivity of developed sensor [84]. This entailed introducing DNA clutch probes, which effectively prevented ssDNA chains from reforming once the DNA double stranded structure was denatured at 90 °C, producing single-stranded DNA. A clamp created to match the wild type equivalent was added to preserve the single-stranded structure of the mutant target ctDNA. The wild type ssDNA sequence is the only one to which this clamp binds. The ctDNA containing the mutations was then able to interact with PNA probe immobilized onto the nanostructured microelectrodes. The voltammetric technique DPV was exploited to quantify the signals produced by unique biosensor. The PNA probe’s selective affinity for the target gene’s modified ssDNA was shown by the covalent bonding technique used to attach it to the gold electrode, which caused changes in the electrode’s potential. This sensor demonstrated an excellent ability to distinguish between mutant ctDNA found in samples taken from melanoma and lung cancer sources. In 2015, Nguyen A H used a PNA probe to capture hot-spot mutations (E542K, E545K) and epigenetic changes in the PIK3CA gene [85]. The ultrasensitive method developed by You J et al.is a highly sensitive and selective real-time PCR method that uses PNA and RNase HII to detect KRAS single nucleotide polymorphisms (SNPs) [71]. This innovative method combines PNA clamping PCR and RNase H-dependent PCR techniques, overcoming their individual limitations. The hybrid approach achieved a detection limit of 1 attomolar (aM) and a selectivity of 0.01%. This study validated the method using DNA-spiked plasma samples, cell cultures, and blood samples from cancer patients. This technique also showed potential for use in breath biopsy.
7.5 DNA probe
To identify PIK3CA gene ctDNA in the circulating blood of gastric cancer patients, a strategy involving the fabrication of graphene oxide gold nanoparticles on a glassy carbon electrode was introduced [74]. The probe DNA was immobilized through weak π–π interactions between DNA base pairs. The hybridization of probe DNA and ctDNA leads to a double-stranded DNA that detaches from the surface of the electrode, resulting in an increase in electrical current. The limit of detection (LOD) for this technique was determined to be 1.0 × 10−20 M. This method holds significant potential for real-time detection studies, especially when it comes to testing cancer patient’s blood samples for ctDNA (Fig. 4). In 2021, Yoon J formulated a MoS2nanosheets polymer biosensor by employing polyxanthurenic acid for film functionalization on a MoS2 electrode [86]. Electrochemically polymerized PXA facilitated the creation of a platform suitable for the identifying the oncogene PIK3CA within the blood of gastric cancer patients. Subsequently, the ssDNA probe was directly attached to the nanocomposite (PXA/MoS2). Alterations in the inherent signal resulting from the hybridization of the ssDNA probe with the target DNA were gauged using electrochemical techniques CV and EIS. Remarkably, the LOD for the PIK3CA gene using this polymer-based biosensor was reported to have sensitivity as low as 1.8 × 10−17 M. A novel imprinted electrochemical biosensor was designed for the accurate identification of carbaryl (1-naphthyl methyl carbamate) [87]. In order to fabricate biosensor, a graphene-ionic liquid nano gold nanocomposite consisting of chitosan-Au Pt alloy nanoparticles was coated on a glassy carbon electrode (GCE), after which a carbaryl-imprinted poly(p-aminothiophenol) (p-ATP) film was electrodeposited. While [Fe(CN)6]3−/[Fe(CN)6]4− acted as the electrochemical redox indicator, the CSAuPtNPs and GR-IL-Au composites were crucial for immobilizing the monomer pATP and enhancing the electrochemical response. The sensor demonstrated a detection range of 0.03 µM to 6.0 µM for carbaryl, with a detection limit of 8 nM and a sensitivity of 4.0 µA/µM mm2. The sensor exhibited excellent sensitivity, selectivity and stability for carbaryl, demonstrating its applicability in practical sample determination, with recoveries ranging from 96 to 105%.
Wang H-F et al.introduced a multifunctional enzyme electrode biosensor for ctDNA detection that specifically targets KRAS G12DM mutations [88]. This biosensor employed a dual-enzyme co-amplification strategy utilizing terminal deoxynucleotidyl transferase and RNase HII. The biosensor features a triple-helix molecular switch (THMS) as a recognition and signal transduction probe, while ribonuclease HII (RNase HII) and terminal deoxynucleotidyl transferase (TdT) serve as double enzyme-assisted amplification accelerators. Under the influence of RNase HII, the signal transduction probe (STP) was released and hybridized with the capture probe on the surface of the gold electrode. Subsequently, TdT-facilitated cascade expansion generated a steady DNA dendritic structure, resulting in the formation of electrically active methylene blue molecules. The biosensor exhibited highly sensitive and accurate ctDNA detection capabilities, achieving a remarkable limit of detection down to the 2.4 aM range. Li and co-workers created silicon nanowires array sensors on silicon-on-insulator to identify PIK3CA ctDNA (SOI) [89]. This involved treating the sensor surface with oxygen (O2) plasma, followed by overnight immersion in APTES and ethanol. Specific ssDNA probes were assembled onto the SiNW surface, creating a functional layer for recognition (ssDNA/SINW-array FET). The biosensing experiments utilized blood serum samples from healthy volunteers with serum samples collected and diluted for the detection of PIK3CA E542K ctDNA. The biosensor demonstrated notable advantages in ctDNA detection, exhibiting a detection range from 0.1 fM to 100 pM and an ultralow detection limit of 10 aM, indicating strong linearity. To enhance ctDNA detection accuracy, the authors devised spiny globular (sea urchin) shaped gold nanocrystals (U–Au) through synergistic interactions involving KBr, CTAC, KI, and L-glutathione [81]. These U–Au-altered multi-graphene aerogels were integrated onto a glass carbon electrode. The sensor sought to accomplish target DNA-triggered cyclic amplification. The design involved hybridization of DNA probe 1 containing methylene blue with DNA probe 2 incorporating ferrocene (Fc) to form dsDNA, which was then linked to U-Au through an Au–S bond. The catalytic redox reactions of Fc and MB enhanced the detection signal. This electrochemical biosensor was effectively employed to detect ctDNA associated with KRAS gene mutations linked to CRC in serum samples. The existence of target DNA initiated a sequence of events involving the hybridization of ctDNA with hairpin DNA 1 (H1), leading to an open hairpin structure. This structural change triggered a cascade, causing more methylene blue probes to approach the electrode surface and more Fc probes to move away. This system exhibited a linear electrochemical response ranging from 1 × 10−1 fM to 1 × 106 fM, with a minimum detection threshold of 0.033 fM. Similarly, a novel ratiometric electrochemical biosensor based on CRISPR/Cas12a for KRAS detection was demonstrated. Authors assures the satisfactory performance of this strategy for detection of ctDNA in human serum [90].
In 2022, Liu Fet al. developed a rapid and accurate ctDNA (Epidermal growth factor receptor (EGFR) L858R detection assay utilizing the CRISPR/Cas12a system and nanocomposites MB/Fe3O4@COF/PdAu [75]. The Cas12a-based CRISPR system was utilized for precise ctDNA target identification and single-stranded DNA cleavage. The nanocomposites exhibited excellent catalysis and amplification of signals (Fig. 5). In the absence of target DNA, Cas12a trans-cleavage activity remained silent, preserving single-stranded DNA integrity. In the presence of the target, trans-cleavage cleaved nonspecific single-stranded DNA, resulting in a reduced MB signal. Quantitative ctDNA detection was achieved by monitoring the current change. The electrochemical biosensor displayed a linear range from 10 aM to 100 pM with a detection limit of 3.3 aM, showing its potential for cancer diagnosis and prognosis. A novel approach to the diagnosis of ctDNA, centered on DNA nanostructure transformation and involving base complementary pairing and the concept of bipedal walking DNA, was proposed [91]. The DNA walking track was constructed on a gold electrode surface, featuring cytosine-rich probe A and probe B. Under alkaline conditions, the reaction between the probes could be disrupted, releasing probe B and enabling electrode regeneration. The target ctDNA unfolded probe E, exposing the template sequence. Polymerase-catalyzed polymerization led to the formation of a double-stranded structure containing a cleavage site. Fragmentation generated segments mirroring the target sequence. The process produced individual single-stranded segments. The movement of the walker cut the track of the electrode surface, causing the electrochemically active substance to disengage and weakening the signal. This method involves double amplification through continuous strand displacement amplification (SDA) and walking nano-machines, ensuring exceptionally high sensitivity in ctDNA diagnosis. Clinical samples for this experiment were collected from healthy individuals and patients diagnosed with breast cancer. The electrochemical response of the biosensor was analyzed using various techniques, which demonstrated a detection limit as low as 2.2 aM.
7.6 Other DNA biosensing methods
Chang et al. introduced a fluorescence polarization technique for ctDNA detection using a chain exchange mechanism and a DNA dissociation probe. This method utilized a toehold-mediated strand displacement reaction (TSDR) to generate fluorescence, allowing qualitative analysis of specific probe-target binding [92]. In 2020, Chen C et al. developed a water soluble positively charged fluorescent probe and demonstrated that the intensity of fluorescence directly correlated with the ctDNA concentration within a specific range. A dual-recognition fluorescent biosensor targeting ctDNA was designed by combining peptide nucleic acid binding and terminal protection of small molecule linked DNA [93]. The biosensor achieved an impressive low detection limit of 0.32pMby targeting the tumor specific PIK3CA gene mutation (E542K) and methylation of the in ctDNA, while maintaining excellent selectivity. Zhai X et al. suggested a novel ctDNA sensing approach using flow cytometry combined with enzyme free amplification with assisted magnetic separation [94]. This technique employs hybridization chain reactions to produce a fluorescent elongated DNA assembly, which is captured by magnetic beads, and fluorescence was measured via flow cytometry. Furthermore, Zhou et al. presented a novel design involving single walled carbon nanotubes (SWCNTs)based on surface enhanced Raman scattering (SERS) coupled with RNase HII aided amplification for acute ctDNA detection in blood, attaining a 3.0 × 10−16 M detection limit for the KRAS G12DM gene (Fig. 6) [95].
Ruimin and co-authors presented a novel amplified colorimetric biosensor by combining the activity of DNAzymes through triplex DNA formation with the hybridization chain reaction amplification for the detection of ctDNA. HCR is triggered by the presence of ctDNA, which results in the production of HCR products that have particular GGG repeat sequences [96]. The catalytic activity (peroxidase) of DNAzymes is enhanced by the synergistic interaction of triplex DNA and asymmetrically split G-quadruplex structures, resulting in the production of unique color signals. Crucially, no HCR occurs when ctDNA is present, guaranteeing minimal background interference. With an amazing detection limit of 0.1 pM, the sensing platform based on colorimetry was utilized for effective screening of ctDNA in human blood plasma. This study carefully combined DNAzymes and nucleic acid amplification by utilizing triplex DNA formation, providing a highly sensitive signal with negligible background noise. In year 2022, an extremely sensitive and selective electrochemical biosensor was developed to investigate DNA for clinical cancer diagnosis [97]. This biosensor used three-dimensional homogeneous carbon architecture (3D-GHC600) similar to that of graphene and was loaded with gold-platinum (AuPt). Cu-BTC, a copper-based metal-organic framework, was annealed at an ideal temperature of 600 °C to produce AuPt. AuPt/3D-GHC600 is the resultant composite catalyst with remarkable electrochemical characteristics. The biosensor creates a sandwich-like structure by fabricating layer-by-layer capture probes (CPs), target DNA (tDNA), and signal probes (SPs) on the electrode by using this catalyst as a label for the SPs. The biosensor exhibited exceptional performance with a detection limit of 2.25 × 10−18 M (S/N = 3) and a wide linear range of 10−8 M to 10−17 M.
7.7 RNA probe
Modified dCas9–sgRNA-based label-free biosensors that was integrated by Uygun ZO et al., into lab-on-a-chip platforms to offer a significant benefits in the field of biosensors [98]. These technologies enabled quick and affordable measurements with small sample volumes. This work presented a novel approach for the label-free detection of ctDNAs using synthetic guide RNA (sgRNA) as the bio recognition receptor and graphene oxide screen-printed electrodes (GPHOXE) modified with deactivated Cas9 (dCas9) proteins. By using electrochemical impedance spectroscopy (EIS) analysis for sequence-specific recognition, the biosensor specifically targeted a tumor-linked mutation (PIK3CA exon 9 mutation). High specificity, as shown by the lack of impedance signals for other ctDNA sequences, including those with single nucleotide mismatches, is one of the noteworthy characteristics of ctDNA. The dCas9-sgRNA altered biosensor exhibited a linear detection range of 2 to 20 nM in just 40 s for 120 bp ctDNA. The biosensor’s effectiveness is highlighted by its linear calibration curve, low detection limit of 0.65 nM, and limit of quantification of 1.92 nM. Research on selectivity and repeatability using actual blood samples has shown a recovery rate of more than 96%. In conclusion, the effective immobilization and optimization of dCas9-sgRNA on GPHOXE show the promise of this CRISPR-dCas9-driven impedimetric system, a ground-breaking achievement in the field of label-free ctDNA detection using an impedimetric technique.
Authors reported the efficacy of circulating microRNAs (miRNAs) as robust cancer biomarkers due to their high stability in body fluids, facilitating non-invasive detection [13]. Addressing the critical need for portable, cost-effective biosensing systems for early on-site diagnosis, particularly in remote areas with limited resources, this innovative system consisted of a disposable screen-printed biosensor altered using a reduced graphene oxide/gold (rGO/Au) composite. Remarkably, in the concentration range of 1 × 10−4 M to 1 × 10−12 M, the smartphone-based biosensing system demonstrated results comparable to those of a commercial electrochemical workstation for miR-21 detection, demonstrating a strong 0.99 correlation coefficient (r2). The biosensing system not only exhibited superior selectivity in identifying mismatched and non-target sequences but also attained a satisfactory recovery rate in artificial saliva, ranging from 96.2 to 107.2%. In 2016, the potential of miRNAs was explored as reliable biomarkers for early-stage breast cancer detection. Serum samples from a large population, including breast cancer patients and various control groups, were comprehensively analyzed using highly sensitive microarray technology [99]. The data were divided into training and test cohorts, with the former utilized to determine a miRNA combination indicative of breast cancer. Subsequently, the test cohort was used to validate the effectiveness of this miRNA combination. This study identified a promising set of miRNAs capable of detecting breast cancer with a high sensitivity of 97.3%, specificity of 82.9%, and overall precision of 89.7%. These findings suggest the potential therapeutic benefit of serum miRNA profiling to ensure precision and early breast cancer diagnosis. These studies highlighted innovative approaches and advancements in biosensor technology and personalized cancer monitoring assays, contributing valuable insights to the field of molecular diagnostics (Fig. 7).
7.8 Antibody probe-based diagnosis
Immunoglobulin have gained widespread application in disease detection, management, food safety control, and environmental monitoring [100]. The foundation of detecting pathogenic bacteria, micro molecules, cells, microbes, and other bioactive entities relies upon the precise binding facilitated by antibodies. Employing antibody probes for detection offers distinctive advantages, particularly in reducing nonspecific interference and achieving heightened sensitivity at lower detection thresholds [101]. In the quest for ctDNA detection, electrodes are outfitted with antibodies designed for specific interactions with ctDNA. Subsequently, the immobilized ctDNA is identified by employing either an electrochemical methodology or an enzyme-linked immunosorbent assay. In the current context, site-specific methylation of DNA has gained prominence as a biomarker. Furthermore, ctDNA methylation serves as a critical epigenetic modification in tumor control, and assessing the methylation levels in ctDNA can be used to determine how dangerous a malignant tumor is. Methods for ctDNA methylation analysis typically include PCR, microarray, sequencing, and others. However, all these approaches necessitate ctDNA pre-treatment [102]. Conversely, the direct immobilization of 5-methylcytosine (5-mC) monoantibodies onto electrodes through covalent coupling enables the capture of methylated ctDNA without the need for sample pre-treatment. Povedano E et al., proposed employing a single 5-mC antibody as a receptor for ctDNA and utilizing a hydrogen peroxide/hydroquinone system to modify screen-printed carbon electrodes (SPECs) for amperometric testing [103]. They proposed two biosensor configurations, one utilizing anti-5-mC as the bioreceptor for sandwich immunoassay and the other employing a specific DNA probe and anti-5-mC as bioreceptors to detect methylated DNA. Notably, DNA probes and anti-5-mC sensors displayed sensitivity in detecting bioreceptors and gene-specific methylation. successfully developed a ctDNA biosensor that confirmed the presence of RNA methylation with a lower limit of 1.25 × 10−15 M without altering the modified antibody on the sensor [104]. Parallel to conventional techniques, the antigen is physically absorbed by the sensor electrode, offering simplicity of operation, cost-effectiveness, and the ability to specifically identify ctDNA methylation. Consequently, this sensor approach is suitable for real-time monitoring and mobile healthcare applications. The immunosensor used two different types of antibodies. First, a 5-mC primary antibody was used to capture any single-stranded DNA with a ctDNA methylation sequence by becoming immobilized on the surface of magnetic beads modified with carboxylic acid. The secondary antibody was labeled with peroxidase (HRP/anti/ssDNA), which served as a motioning antibody to recognize a small amount of single-stranded DNA.
7.9 Limitations and challenges of ctDNA diagnosis
To effectively exploit ctDNA as a cancer molecular marker in clinical diagnosis, reliable and accurate measurement approaches are crucial. Blood plasma ctDNA levels are generally low in solid tumor malignancies, with mutant allele fractions in locally advanced non-metastatic disease typically less than 1% and in advanced metastatic disease typically less than 10%. These levels decrease further in early-stage cancers and after curative-intent treatment, where mutant allele fractions can be less than 0.1%. Highly sensitive methods are therefore necessary for pre-treatment and post-treatment ctDNA measurements [105]. A scarcity of clinical samples may diminish the quantity of circulating free DNA (cfDNA) molecules available for assessment in cancer patients with health conditions such as anemia, low white blood cells, high cholesterol, and poor performance status, thereby complicating ctDNA detection. Various processes lead to the release of ctDNA, including senescence, active secretion in extracellular vesicles (EVs), mitochondrial DNA (mtDNA) discharge, tumor necrosis, cell death by iron accumulation (ferroptosis), cell bursts due to cytoplasmic swelling (oncosis), proinflammatory cell rupture (pyroptosis), phagocytosis and programmed cell death, which subsequently impact ctDNA detection [106].
ctDNA detection can be confounded by clonal haematopoiesis of indeterminate potential (CHIP), where in haematopoietic progenitor cells, age-dependent acquired mutations accumulate and result in a genetically distinct subpopulation [107]. CHIP is most likely to give false-positive results when non reference variants are detected in blood plasma, especially when the ctDNA mutant allele fraction is low in the setting of minimal residual disease (MRD) diagnosis [97, 98]. Focusing on clonal mutations and making use of assays that use paired peripheral blood mononuclear cells (PBMCs) for sequencing can improve specificity in ctDNA detection. Despite the prevalence of CHIP, especially in older individuals, it is crucial for specific ctDNA measurements, particularly when utilizing extremely sensitive assays that are intended to identify variants with low mutant allele fractions [110]. Beyond the CHIP method, other sources of somatic chimeras may introduce possible confounders in ctDNA analysis. Environmental factors and intrinsic errors in DNA replication can cause somatic mosaicism, which can result in different types of genomic alterations. refined methods for detecting ctDNA, such as sequencing donor plasma that matches recipient PBMCs and using the results to filter variants in the cell-free compartment, are essential for accurately identifying somatic mosaicism [105]. Despite promising early results, there are still a number of obstacles preventing the widespread clinical application of ctDNA-based testing in informed adjuvant treatment and tumor surveillance. The application of electrochemical-based ctDNA testing has the potential to broaden its scope to include various other cancer types. Currently, many cancer types remain unexplored by this particular detection method [111]. Serious concerns have been raised about the limited sensitivity of ctDNA assays, particularly in regard to patients who have undergone resection for early-stage colon cancer and have relatively low plasma ctDNA levels. In the early postoperative phase, the estimated sensitivity for a single ctDNA measurement was only 50%. Assay sensitivity may be increased by using larger sample volumes, tracking multiple mutations, fragment size analysis, serial testing, and NGS panels, which allow for the testing of a vast range of genomic and epigenetic modifications of oncogenes and proto-oncogenes.
7.10 Future perspectives
As the family physician industry continues to expand, the use of portable, versatile, and dependable clinical diagnostic tools has increased. With regard to point-of-care testing, electrochemical biosensors made for ctDNA detection have enormous potential [112]. While ctDNA introduces novel markers for cancer identification, various limitations persist in the realm of ctDNA detection through electrochemical biosensors. Currently, existing electrochemical biosensors are restricted to identifying specific known biomarkers. However, clinical diagnoses often require the detection of multiple targets [113], especially in cases involving unknown mutations. Currently, ctDNA detection sensors are not poised for widespread clinical deployment and demand further research. A noteworthy limitation is that most sensors are equipped with only a single detection channel, resulting in low detection throughput. The future trajectory suggests a shift toward array-based, high-throughput sensors integrated with microfluidic systems to overcome this constraint. Additionally, the current landscape of ctDNA detection lacks comprehensive testing for various cancer types, with certain categories such as sarcomas and silent cancers such as ovarian cancers remaining relatively unexplored [114]. However, despite the high cost and intricate nature of the ctDNA test, it is likely to encounter challenges similar to traditional biomarkers, such as low specificity and sensitivity, when used for population screening and the early diagnosis of malignant tumors in asymptomatic individuals.
Given the scant abundance of ctDNA in early-stage cancer and precancerous lesions, sensors must possess both a lower detection threshold and heightened specificity [9]. Notably, the fabrication of nanomaterials on the electrode surface increases the surface area and conductivity, which has been proven to substantially enhance the sensor sensitivity. Despite the significant strides achieved in ctDNA detection via electrochemical sensors, these technologies are not yet poised to fully supplant traditional tumor imaging methods. Consequently, it remains essential to merge biosensors with other analytical techniques for comprehensive insights, thereby minimizing any potential harm to patients during sampling and diagnostic procedures [115].
8 Conclusions
This review explores recent strides in the development of electrochemical biosensors designed for detecting ctDNA. Despite the conventional use of PCR and DNA sequencing in clinical settings for ctDNA detection, the drawbacks of these biosensors, such as costly equipment, intricate procedures, frequent false positives and low sensitivity, limit their applicability for point-of-care diagnostics. To overcome these challenges, the advent of sensitive and specific biosensors has emerged as a promising solution. Cancer-associated ctDNA often exhibits high mutation frequencies in the TP53, HER-2, KRAS, and EGFR genes, surpassing a 50% alteration frequency. Identifying these prevalent mutations in metastatic cancers has potential for identifying patients with premalignant tumors and predicting cancer progression. Analyzing ctDNA provides valuable insights for prognosis and facilitates tailored treatment selection. However, challenges in ctDNA detection and analysis must be addressed before widespread adoption, emphasizing the need for additional clinical trials to evaluate the significance of ctDNA in prevalent malignancies. With ongoing advancements in personalized anti-neoplastic therapies, further exploration is warranted to detect panels of ctDNA and patient-specific ctDNA across diverse cancer types. Conventional ctDNA detection is limited by the significant drawback of requiring a large blood volume to detect trace amounts of ctDNA due to its low limit of detection (LOD). This limitation can be addressed by substituting it with an electrochemical biosensor, which boasts a higher LOD. These biosensors streamline operations, enabling real-time monitoring and positioning as potent tools for ctDNA detection. Their portability and cost-effectiveness make them pivotal components in the evolving technological landscape for ctDNA detection and point-of-care testing.
Data availability
No datasets were generated or analysed during the current study.
References
Frick C, Rumgay H, Vignat J, Ginsburg O, Nolte E, Bray F, et al. Quantitative estimates of preventable and treatable deaths from 36 cancers worldwide: a population-based study. Lancet Glob Heal. 2023;11(11):e1700–12.
McLendon R, Bigner D, Friedman A, Meir G, Van E, Mastrogianakis GM, Olson JJ, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8.
Rodríguez J, Avila J, Rolfo C, Ruíz-Patiño A, Russo A, Ricaurte L, et al. When tissue is an issue the Liquid Biopsy is Nonissue: a review. Oncol Ther. 2021;9(1):89–110.
Gilson P, Merlin J-L, Harlé A. Deciphering Tumour Heterogeneity: from tissue to Liquid Biopsy. Cancers (Basel). 2022;14(6):1384.
Cecchini MJ, Yi ES. Liquid biopsy is a valuable tool in the diagnosis and management of lung cancer. J Thorac Dis. 2020;12(11):7048–56.
Garzarelli V, Ferrara F, Primiceri E, Chiriacò MS. Biofluids manipulation methods for liquid biopsy in minimally-invasive assays. MethodsX. 2022;9:101759.
Corvigno S, Johnson AM, Wong K-K, Cho MS, Afshar-Kharghan V, Menter DG, et al. Novel markers for Liquid biopsies in Cancer Management: circulating platelets and extracellular vesicles. Mol Cancer Ther. 2022;21(7):1067–75.
Biglari N, Soltani-Zangbar MS, Mohammadian J, Mehdizadeh A, Abbasi K. ctDNA as a novel and promising approach for cancer diagnosis: a focus on hepatocellular carcinoma. EXCLI J. 2023;22:752–80.
Farshchi F, Hasanzadeh M. Efficient diagnosis of cancer using biosensing of circulating tumor DNAs(ctDNA): recent progress and challenges. Microchem J. 2023;193:109076.
Huang A, Zhang X, Zhou S-L, Cao Y, Huang X-W, Fan J, et al. Detecting circulating Tumor DNA in Hepatocellular Carcinoma patients using Droplet Digital PCR is feasible and reflects Intratumoral Heterogeneity. J Cancer. 2016;7(13):1907–14.
Gauri S, Ahmad MR. ctDNA detection in Microfluidic platform: a Promising Biomarker for Personalized Cancer Chemotherapy. J Sens. 2020;2020:1–10.
Armakolas A, Kotsari M, Koskinas J. Liquid biopsies, novel approaches and future directions. Cancers (Basel). 2023;15(5):1579.
Shin Low S, Pan Y, Ji D, Li Y, Lu Y, He Y, et al. Smartphone-based portable electrochemical biosensing system for detection of circulating microRNA-21 in saliva as a proof-of-concept. Sens Actuators B Chem. 2020;308:127718.
Filik H, Avan AA. Nanostructures for nonlabeled and labeled electrochemical immunosensors: simultaneous electrochemical detection of cancer markers: a review. Talanta. 2019;205:120153.
Cheng F, Su L, Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget. 2016;7(30):48832–41.
Benesova L, Belsanova B, Suchanek S, Kopeckova M, Minarikova P, Lipska L, et al. Mutation-based detection and monitoring of cell-free tumor DNA in peripheral blood of cancer patients. Anal Biochem. 2013;433(2):227–34.
Werner B, Warton K, Ford CE. Transcending Blood—opportunities for alternate liquid biopsies in Oncology. Cancers (Basel). 2022;14(5):1309.
Khier S, Gahan PB. Hepatic clearance of cell-free DNA: possible impact on early metastasis diagnosis. Mol Diagn Ther. 2021;25(6):677–82.
Zhu J, Huang J, Zhang P, Li Q, Kohli M, Huang C-C, et al. Advantages of single-stranded DNA over double-stranded DNA Library Preparation for capturing cell-free tumor DNA in plasma. Mol Diagn Ther. 2020;24(1):95–101.
Shegekar T, Vodithala S, Juganavar A. The emerging role of Liquid biopsies in Revolutionising Cancer diagnosis and therapy. Cureus. 2023. https://doi.org/10.7759/cureus.43650.
Diehl F, Li M, He Y, Kinzler KW, Vogelstein B, Dressman D. BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat Methods. 2006;3(7):551–9.
O’Leary B, Hrebien S, Beaney M, Fribbens C, Garcia-Murillas I, Jiang J, et al. Comparison of BEAMing and Droplet Digital PCR for circulating tumor DNA analysis. Clin Chem. 2019;65(11):1405–13.
Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N et al. Detection of circulating Tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224).
Ma F, Zhu W, Guan Y, Yang L, Xia X, Chen S, et al. ctDNA dynamics: a novel indicator to track resistance in metastatic breast cancer treated with anti-HER2 therapy. Oncotarget. 2016;7(40):66020–31.
Ortiz - Cuaran S, Mezquita L, Swalduz A, Aldea M, Mazieres J, Jovelet C, et al. Circulating tumour DNA (ctDNA) analysis depicts mechanisms of resistance and tumour response to BRAF inhibitors in BRAF-mutant non-small cell lung cancer (NSCLC). Ann Oncol. 2019;30:v641.
Anandappa G, Starling N, Begum R, Bryant A, Sharma S, Renner D, et al. Minimal residual disease (MRD) detection with circulating tumor DNA (ctDNA) from personalized assays in stage II-III colorectal cancer patients in a U.K. multicenter prospective study (TRACC). J Clin Oncol. 2021;39(3suppl):102–102.
Ricciuti B, Jones G, Severgnini M, Alessi JV, Recondo G, Lawrence M, et al. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC). J Immunother Cancer. 2021;9(3):e001504.
Schwaederlé MC, Patel SP, Husain H, Ikeda M, Lanman RB, Banks KC, et al. Utility of genomic Assessment of blood-derived circulating tumor DNA (ctDNA) in patients with Advanced Lung Adenocarcinoma. Clin Cancer Res. 2017;23(17):5101–11.
Cabel L, Jeannot E, Bieche I, Vacher S, Callens C, Bazire L, et al. Prognostic impact of residual HPV ctDNA detection after Chemoradiotherapy for anal squamous cell carcinoma. Clin Cancer Res. 2018;24(22):5767–71.
Tie J, Cohen J, Wang Y, Li L, Kinde I, Elsaleh H, et al. The potential of circulating tumor DNA (ctDNA) to guide adjuvant chemotherapy decision making in locally advanced rectal cancer (LARC). J Clin Oncol. 2017;35(15suppl):3521–3521.
Cavallone L, Aguilar A, Aldamry M, Lafleur J, Brousse S, Lan C, et al. Circulating tumor DNA (ctDNA) during and after neoadjuvant chemotherapy and prior to surgery is a powerful prognostic factor in triple-negative breast cancer (TNBC). J Clin Oncol. 2019;37(15suppl):594–594.
van Ginkel JH, Huibers MMH, van Es RJJ, de Bree R, Willems SM. Droplet digital PCR for detection and quantification of circulating tumor DNA in plasma of head and neck cancer patients. BMC Cancer. 2017;17(1):428.
Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7(302).
Leal A, van Grieken NCT, Palsgrove DN, Phallen J, Medina JE, Hruban C, et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat Commun. 2020;11(1):525.
Charo LM, Eskander RN, Okamura R, Patel SP, Nikanjam M, Lanman RB, et al. Clinical implications of plasma circulating tumor DNA in gynecologic cancer patients. Mol Oncol. 2021;15(1):67–79.
Moss EL, Gorsia DN, Collins A, Sandhu P, Foreman N, Gore A, et al. Utility of circulating Tumor DNA for detection and monitoring of Endometrial Cancer recurrence and progression. Cancers (Basel). 2020;12(8):2231.
Herzog H, Dogan S, Aktas B, Nel I. Targeted sequencing of plasma-derived vs. urinary cfDNA from patients with triple-negative breast Cancer. Cancers (Basel). 2022;14(17).
Faaborg L, Fredslund Andersen R, Waldstrøm M, Høgdall E, Høgdall C, Adimi P, et al. Analysis of HOXA9 methylated ctDNA in ovarian cancer using sense-antisense measurement. Clin Chim Acta. 2021;522:152–7.
Azad TD, Chaudhuri AA, Fang P, Qiao Y, Esfahani MS, Chabon JJ, et al. Circulating tumor DNA analysis for detection of minimal residual Disease after Chemoradiotherapy for localized esophageal Cancer. Gastroenterology. 2020;158(3):494–e5056.
Chen S, Zhao J, Cui L, Liu Y. Urinary circulating DNA detection for dynamic tracking of EGFR mutations for NSCLC patients treated with EGFR-TKIs. Clin Transl Oncol. 2017;19(3):332–40.
Husain H, Melnikova VO, Kosco K, Woodward B, More S, Pingle SC, et al. Monitoring Daily Dynamics of Early Tumor Response to targeted therapy by detecting circulating Tumor DNA in urine. Clin Cancer Res. 2017;23(16):4716–23.
Yi X, Ma J, Guan Y, Chen R, Yang L, Xia X. The feasibility of using mutation detection in ctDNA to assess tumor dynamics. Int J Cancer. 2017;140(12):2642–7.
Lin C, Liu X, Zheng B, Ke R, Tzeng C-M. Liquid biopsy, ctDNA diagnosis through NGS. Life. 2021;11(9):890.
Williams CG, Lee HJ, Asatsuma T, Vento-Tormo R, Haque A. An introduction to spatial transcriptomics for biomedical research. Genome Med. 2022;14(1):68.
Wang Y, Yang Q, Wang Z. The evolution of nanopore sequencing. Front Genet. 2015;5.
Marcozzi A, Jager M, Elferink M, Straver R, van Ginkel JH, Peltenburg B, et al. Accurate detection of circulating tumor DNA using nanopore consensus sequencing. npj Genomic Med. 2021;6(1):106.
Aparicio-Puerta E, Jáspez D, Lebrón R, Koppers-Lalic D, Marchal JA, Hackenberg M. liqDB: a small-RNAseq knowledge discovery database for liquid biopsy studies. Nucleic Acids Res. 2019;47(D1):D113–20.
Bos MK, Nasserinejad K, Jansen MPHM, Steendam CMJ, Angus L, Atmodimedjo PN, et al. Comparison of variant allele frequency and number of mutant molecules as units of measurement for circulating tumor DNA. Mol Oncol. 2021;15(1):57–66.
Aparicio-Puerta E, Lebrón R, Rueda A, Gómez-Martín C, Giannoukakos S, Jaspez D, et al. sRNAbench and sRNAtoolbox 2019: intuitive fast small RNA profiling and differential expression. Nucleic Acids Res. 2019;47(W1):W530–5.
Rose Brannon A, Jayakumaran G, Diosdado M, Patel J, Razumova A, Hu Y, et al. Enhanced specificity of clinical high-sensitivity tumor mutation profiling in cell-free DNA via paired normal sequencing using MSK-ACCESS. Nat Commun. 2021;12(1):3770.
Klega K, Imamovic-Tuco A, Ha G, Clapp AN, Meyer S, Ward A et al. Detection of somatic structural variants enables quantification and characterization of circulating Tumor DNA in children with solid tumors. JCO Precis Oncol. 2018;(2):1–13.
Zhao J, Reuther J, Scozzaro K, Hawley M, Metzger E, Emery M, et al. Personalized Cancer monitoring assay for the detection of ctDNA in patients with solid tumors. Mol Diagn Ther. 2023;27(6):753–68.
Naresh V, Lee N. A review on biosensors and recent development of Nanostructured materials-enabled biosensors. Sensors. 2021;21(4):1109.
Barhoum A, Hamimed S, Slimi H, Othmani A, Abdel-Haleem FM, Bechelany M. Modern designs of electrochemical sensor platforms for environmental analyses: principles, nanofabrication opportunities, and challenges. Trends Environ Anal Chem. 2023;38:e00199.
Zhang W, Wang R, Luo F, Wang P, Lin Z. Miniaturized electrochemical sensors and their point-of-care applications. Chin Chem Lett. 2020;31(3):589–600.
Perumal V, Hashim U. Advances in biosensors: Principle, architecture and applications. J Appl Biomed. 2014;12(1):1–15.
Mahshid SS, Flynn SE, Mahshid S. The potential application of electrochemical biosensors in the COVID-19 pandemic: a perspective on the rapid diagnostics of SARS-CoV-2. Biosens Bioelectron. 2021;176:112905.
Manshadi MKD, Mansoorifar A, Chiao J-C, Beskok A. Impedance-based neutralizing antibody detection Biosensor with Application in SARS-CoV-2 infection. Anal Chem. 2023. https://doi.org/10.1021/acs.analchem.2c03193.
Ahirwar R. Recent advances in nanomaterials-based electrochemical immunosensors and aptasensors for HER2 assessment in breast cancer. Microchim Acta. 2021;188(10):317.
Ronkainen NJ, Halsall HB, Heineman WR. Electrochemical biosensors. Chem Soc Rev. 2010;39(5):1747.
Cohen SA, Liu MC, Aleshin A. Practical recommendations for using ctDNA in clinical decision making. Nature. 2023;619(7969):259–68.
Beeram R, Vepa KR, Soma VR. Recent trends in SERS-Based Plasmonic Sensors for Disease Diagnostics, Biomolecules Detection, and machine learning techniques. Biosensors. 2023;13(3):328.
Sumitha MS, Xavier TS. Recent advances in electrochemical biosensors – A brief review. Hybrid Adv. 2023;2:100023.
Viswanathan S, Radecka H, Radecki J. Electrochemical biosensors for food analysis. Monatshefte für Chemie - Chem Mon. 2009;140(8):891–9.
Bakirhan NK, Topal BD, Ozcelikay G, Karadurmus L, Ozkan SA. Current advances in Electrochemical biosensors and nanobiosensors. Crit Rev Anal Chem. 2022;52(3):519–34.
Kimmel DW, LeBlanc G, Meschievitz ME, Cliffel DE. Electrochemical Sensors and biosensors. Anal Chem. 2012;84(2):685–707.
Hammond JL, Formisano N, Estrela P, Carrara S, Tkac J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016;60(1):69–80.
LICHTENSTEIN AV, MELKONYAN HS, TOMEI LD. Circulating nucleic acids and apoptosis. Ann N Y Acad Sci. 2001;945(1):239–49.
Karimi F, Karimi-Maleh H, Rouhi J, Zare N, Karaman C, Baghayeri M, et al. Revolutionizing cancer monitoring with carbon-based electrochemical biosensors. Environ Res. 2023;239:117368.
Chabbah T, Abderrazak H, Souissi R, Saint-Martin P, Casabianca H, Chatti S, et al. A sensitive Impedimetric Sensor based on Biosourced Polyphosphine films for the detection of lead ions. Chemosensors. 2020;8(2):34.
You J, Jang K, Park H, Lee S, Lim A, Park C, et al. Ultrahigh sensitive and selective detection of single nucleotide polymorphism using peptide nucleic acid and ribonuclease H assembled DNA amplification (PRADA). Anal Chim Acta. 2022;1233:340423.
Yuan L, Liu L. Peptide-based electrochemical biosensing. Sens Actuators B Chem. 2021;344:130232.
Cai C, Guo Z, Cao Y, Zhang W, Chen Y. A dual biomarker detection platform for quantitating circulating tumor DNA (ctDNA). Nanotheranostics. 2018;2(1):12–20.
Rahman M, Cui D, Zhou S, Zhang A, Chen D. A graphene oxide coated gold nanostar based sensing platform for ultrasensitive electrochemical detection of circulating tumor DNA. Anal Methods. 2020;12(4):440–7.
Liu F, Peng J, Lei Y-M, Liu R-S, Jin L, Liang H, et al. Electrochemical detection of ctDNA mutation in non-small cell lung cancer based on CRISPR/Cas12a system. Sens Actuators B Chem. 2022;362:131807.
Tang S, Xu Q, Liu M, Zhu Y, Zhang G, Tang X. Highly sensitive electrochemical immunosensor based on SiO2 nanospheres for detection of EGFR as colorectal cancer biomarker. Alexandria Eng J. 2024;89:53–9.
Jafari-Kashi A, Rafiee-Pour H-A, Shabani-Nooshabadi M. A new strategy to design label-free electrochemical biosensor for ultrasensitive diagnosis of CYFRA 21–1 as a biomarker for detection of non-small cell lung cancer. Chemosphere. 2022;301:134636.
Cai A, Yang L, Kang X, Liu J, Wang F, Ji H, et al. Target Recognition– and HCR amplification–Induced in situ Electrochemical Signal Probe Synthesis Strategy for Trace ctDNA Analysis. Biosensors. 2022;12(11):989.
Rahman M, Niu J, Cui X, Zhou C, Tang N, Jin H, et al. Electrochemical Biosensor based on < scp > l -Arginine and rGO-AuNSs deposited on the Electrode Combined with DNA Probes for Ultrasensitive Detection of the gastric Cancer-related PIK3CA gene of ctDNA. ACS Appl Bio Mater. 2022;5(11):5094–103.
Zhang W, Dai Z, Liu X, Yang J. High-performance electrochemical sensing of circulating tumor DNA in peripheral blood based on poly-xanthurenic acid functionalized MoS 2 nanosheets. Biosens Bioelectron. 2018;105:116–20.
Yuanfeng P, Ruiyi L, Xiulan S, Guangli W, Zaijun L. Highly sensitive electrochemical detection of circulating tumor DNA in human blood based on urchin-like gold nanocrystal-multiple graphene aerogel and target DNA-induced recycling double amplification strategy. Anal Chim Acta. 2020;1121:17–25.
Yang J, Yin X, Zhang W. Electrochemical determination of PIK3CA gene associated with breast cancer based on molybdenum disulfide nanosheet-supported poly(indole-6-carboxylic acid). Anal Methods. 2019;11(2):157–62.
Chu Y, Cai B, Ma Y, Zhao M, Ye Z, Huang J. Highly sensitive electrochemical detection of circulating tumor DNA based on thin-layer MoS 2 /graphene composites. RSC Adv. 2016;6(27):22673–8.
Das J, Ivanov I, Sargent EH, Kelley SO. DNA Clutch Probes for circulating tumor DNA analysis. J Am Chem Soc. 2016;138(34):11009–16.
Nguyen AH, Sim SJ. Nanoplasmonic biosensor: detection and amplification of dual bio-signatures of circulating tumor DNA. Biosens Bioelectron. 2015;67:443–9.
Yoon J, Lim J, Shin M, Lee S-N, Choi J-W. Graphene/MoS2 Nanohybrid for biosensors. Mater (Basel). 2021;14(3):518.
Zhao L, Zhao F, Zeng B. Electrochemical Determination of Carbaryl by using a molecularly imprinted Polymer/Graphene-Ionic liquid-Nano Au/chitosan-AuPt Alloy nanoparticles Composite Film Modified Electrode. Int J Electrochem Sci. 2014;9(3):1366–77.
Wang H-F, Ma R-N, Sun F, Jia L-P, Zhang W, Shang L, et al. A versatile label-free electrochemical biosensor for circulating tumor DNA based on dual enzyme assisted multiple amplification strategy. Biosens Bioelectron. 2018;122:224–30.
Li D, Chen H, Fan K, Labunov V, Lazarouk S, Yue X, et al. A supersensitive silicon nanowire array biosensor for quantitating tumor marker ctDNA. Biosens Bioelectron. 2021;181:113147.
Dong J, Li X, Hou C, Hou J, Huo D. A novel CRISPR/Cas12a-Mediated Ratiometric Dual-Signal Electrochemical Biosensor for Ultrasensitive and Reliable Detection of circulating Tumor deoxyribonucleic acid. Anal Chem. 2024;96(18):6930–9.
Miao P, Chai H, Tang Y. DNA hairpins and dumbbell-wheel transitions amplified walking Nanomachine for Ultrasensitive Nucleic Acid Detection. ACS Nano. 2022;16(3):4726–33.
Chang H, Zhang Y, Yang F, Wang C, Dong H. ctDNA detection based on DNA clutch probes and strand exchange mechanism. Front Chem. 2018;6(OCT):1–7.
Chen C, He R, Zhang Z, Chen Y. Dual-recognition-based determination of ctDNA via the clamping function of peptide nucleic acid and terminal protection of small-molecule-linked DNA. Analyst. 2020;145(23):7603–8.
Zhai X, Li J, Cao Y, Zhu X, Tang Y, Chen G, et al. A nanoflow cytometric strategy for sensitive ctDNA detection via magnetic separation and DNA self-assembly. Anal Bioanal Chem. 2019;411(23):6039–47.
Zhou Q, Zheng J, Qing Z, Zheng M, Yang J, Yang S, et al. Detection of circulating Tumor DNA in human blood via DNA-Mediated surface-enhanced Raman Spectroscopy of single-walled Carbon nanotubes. Anal Chem. 2016;88(9):4759–65.
Li R, Zou L, Luo Y, Zhang M, Ling L. Ultrasensitive colorimetric detection of circulating tumor DNA using hybridization chain reaction and the pivot of triplex DNA. Sci Rep. 2017;7.
Chen KC, Zhao HL, Wang ZX, Lan MB. Three-dimensional graphene-like homogeneous carbon architecture loaded with gold-platinum for the electrochemical detection of circulating tumor DNA. Mater Today Chem. 2022;24:100892.
Uygun ZO, Yeniay L, Gi̇rgi̇n Sağın F. CRISPR-dCas9 powered impedimetric biosensor for label-free detection of circulating tumor DNAs. Anal Chim Acta. 2020;1121:35–41.
Shimomura A, Shiino S, Kawauchi J, Takizawa S, Sakamoto H, Matsuzaki J, et al. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci. 2016;107(3):326–34.
Di Nardo F, Chiarello M, Cavalera S, Baggiani C, Anfossi L. Ten years of lateral Flow Immunoassay technique applications: trends, challenges and Future perspectives. Sensors. 2021;21(15):5185.
Damborska D, Bertok T, Dosekova E, Holazova A, Lorencova L, Kasak P, et al. Nanomaterial-based biosensors for detection of prostate specific antigen. Microchim Acta. 2017;184(9):3049–67.
Wen X, Pu H, Liu Q, Guo Z, Luo D. Circulating Tumor DNA—A Novel Biomarker of Tumor Progression and its favorable detection techniques. Cancers (Basel). 2022;14(24):6025.
Povedano E, Vargas E, Montiel VRV, Torrente-Rodríguez RM, Pedrero M, Barderas R, et al. Electrochemical affinity biosensors for fast detection of gene-specific methylations with no need for bisulfite and amplification treatments. Sci Rep. 2018;8(1):1–11.
Povedano E, Montiel VR-V, Valverde A, Navarro-Villoslada F, Yáñez-Sedeño P, Pedrero M, et al. Versatile Electroanalytical Bioplatforms for simultaneous determination of Cancer-related DNA 5-Methyl- and 5-Hydroxymethyl-cytosines at global and gene-specific levels in human serum and tissues. ACS Sens. 2019;4(1):227–34.
Chin R-I, Chen K, Usmani A, Chua C, Harris PK, Binkley MS, et al. Detection of solid tumor molecular residual disease (MRD) using circulating tumor DNA (ctDNA). Mol Diagn Ther. 2019;23(3):311–31.
Sánchez-Herrero E, Serna-Blasco R, Robado de Lope L, González-Rumayor V, Romero A, Provencio M. Circulating tumor DNA as a Cancer Biomarker: an overview of Biological features and factors that may Impact on ctDNA Analysis. Front Oncol. 2022;12.
Schmidt A. Clonal hematopoiesis of Indeterminate potential: New insights from recent studies. Heal TIMES Oncol Hematol. 2021. (9.
Hu Y, Ulrich BC, Supplee J, Kuang Y, Lizotte PH, Feeney NB, et al. False-positive plasma genotyping due to Clonal Hematopoiesis. Clin Cancer Res. 2018;24(18):4437–43.
Arisi MF, Dotan E, Fernandez SV. Circulating Tumor DNA in Precision Oncology and its applications in Colorectal Cancer. Int J Mol Sci. 2022;23(8):4441.
Swanton C, Venn O, Aravanis A, Hubbell E, Maddala T, Beausang JF, et al. Prevalence of clonal hematopoiesis of indeterminate potential (CHIP) measured by an ultra-sensitive sequencing assay: exploratory analysis of the circulating Cancer Genome Atlas (CCGA) study. J Clin Oncol. 2018;36(15suppl):12003–12003.
Constantin N, Sina AAI, Korbie D, Trau M. Opportunities for Early Cancer detection: the rise of ctDNA methylation-based Pan-cancer Screening technologies. Epigenomes. 2022;6(1):6.
Barhoum A, Altintas Z, Devi KSS, Forster RJ. Electrochemiluminescence biosensors for detection of cancer biomarkers in biofluids: principles, opportunities, and challenges. Nano Today. 2023;50:101874.
Timilsina SS, Jolly P, Durr N, Yafia M, Ingber DE. Enabling multiplexed Electrochemical detection of biomarkers with high sensitivity in Complex Biological samples. Acc Chem Res. 2021;54(18):3529–39.
Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with emphasis on early detection. BMC Med. 2018;16(1):166.
Tang Z, Huang J, He H, Ma C, Wang K. Contributing to liquid biopsy: Optical and electrochemical methods in cancer biomarker analysis. Coord Chem Rev. 2020;415:213317.
Acknowledgements
Not applicable.
Funding
No external funding.
Author information
Authors and Affiliations
Contributions
SK&RPwas responsible for the writing of the original draft.DK, RN&YD was responsible for the editing of the manuscript.SD, BSwas responsible for material preparation and data collection. NKK&MSNwas responsible for the review of the manuscript.DK, SG, AK was responsible for theconceptualization, final editing and review of the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kumar, S., Poria, R., Kala, D. et al. Recent advances in ctDNA detection using electrochemical biosensor for cancer. Discov Onc 15, 517 (2024). https://doi.org/10.1007/s12672-024-01365-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01365-7