Abstract
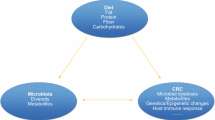
Short-chain fatty acids (SCFAs) are produced by bacterial fermentation in the colon and are thought to be protective against gastrointestinal disease. SCFAs such as acetate, propionate and butyrate are important metabolites in the maintenance of intestinal homeostasis and have been shown to be beneficial in colorectal cancer (CRC). SCFAs are responsible for maintaining a normal intestinal barrier and exhibit numerous immunomodulatory functions. In this review article, we will discuss the metabolism and mechanism of action of SCFAs and their effects on the CRC, with particular emphasis on dietary fiber treatment and the clinical research progress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Short-chain fatty acids (SCFAs) are substances released during the bacterial fermentation of dietary fiber in the gut [1, 2]. In the human body, the most abundant SCFA (≥ 95%) are acetate, propionate and butyrate; their molar ratio is approximately 6:2:2 [3]. Other SCFAs such as formate, valerate and caproate are present in the body in much smaller amounts. Small amounts of SCFA are obtained directly from food, but their main source is fermentation of dietary fiber in the colon [4]. Each day, about 500–600 mmol SCFA is produced in the intestine, however, this value is dependent on many factors, such as the amount of fiber supplied, intestinal transit time, and the composition of the intestinal microbiota. The biological functions of SCFAs include reducing the pH of colon to inhibit the growth of destructive bacteria, and regulating energy metabolism, inflammation, and tumor growth and development [5]. Besides, SCFAs also aid in managing immune regulation, appetite regulation, lipid metabolism and glucose metabolism [6, 7].
2 Relationship between SCFAs and CRC
Colorectal cancer (CRC) is the 3rd most commonly diagnosed cancer (10.0% of the total cases) and the second leading cause of cancer-related mortality (9.4% of the total cancer deaths) in the world [8]. Each year, about 1–2 million new cases of CRC are reported, and 600,000 people die from it. CRC is the most closely related to diet among all cancer types, with 30–50% of colorectal cancer patients being related to diet and nutrition [9]. A large number of epidemiological data and clinical trials have proved that red meat such as beef, pork, and mutton, and processed meat such as sausage and bacon that have been cured and smoked can significantly increase the risk of colorectal cancer [10, 11]. This is an important factor in the increasing incidence of colorectal cancer worldwide. The carcinogenicity of red meat and processed meat may be related to some carcinogens contained in it, and the specific mechanism is not clear. Numerous studies have shown that people who consume more dietary fiber have a relatively low incidence of colorectal cancer, and intake of 10 g of dietary fiber per day can reduce the risk of colorectal cancer by 10%, which is inseparable from the role of SCFAs [12,13,14].
SCFAs are produced by the beneficial bacteria in the microbiome, and they are essential for gut and brain. Butyrate, propionate and acetate are the most abundant SCFAs in the human body. SCFAs significantly improve the function of the intestines, including taking part in maintaining the integrity of the intestinal barrier, protecting against inflammation, increasing mucus production, and stimulating intestinal motility [15,16,17]. Numerous studies suggest their protective and pro-health activity in pathologies of the gastrointestinal tract, such as inflammatory bowel diseases (IBD) and CRC [18,19,20,21].
Butyrate, one of the most important SCFAs, was produced by healthy gut microbiota (including Coprococcus comes, Coprococcus eutactus, Anaerostipes spp., Coprococcus catus, Eubacterium rectale, Eubacterium hallii, Faecalibacterium prausnitzii,Roseburia spp.) [22]. Lactate and acetate may serve as substrates for the production of butyrate [23]. Recent studies have shown that low levels of butyrate are associated with a higher incidence of CRC [24, 25]. In fact, butyrate is foremost source of energy supply and also stimulates mucosal proliferation under certain conditions. When epithelial cells are energy-deficient, butyrate is used for energy supply; when energy is sufficient, butyrate is used to induce DNA-damaged cell differentiation, apoptosis, and inhibit tumor cell proliferation [26, 27]. Ma X [28] also showed that butyrate significantly inhibits liver metastasis of CRC cells, improves intestinal dysbiosis in mice and enhances antitumor immune responses in liver of mice. Although acetate and propionate are not as powerful as butyrate in preventing CRC, they still show good protection in a large number of studies (Table 1).
3 The metabolism and mechanism of SCFAs
The gut microbiota produces SCFAs through fermentation of dietary fiber in colon. The concentration of SCFAs varies according to the section of the colon, which reaches a concentration of approximately 70–140 mM in the proximal part of the colon, and drops to 20–70 mM in the distal part of the colon. The higher concentration of SCFAs in the proximal colon is due to the greater availability of carbohydrates and water in this part of the intestine. The difference in the concentration of SCFAs means that the pH value is different along the human colon [4].
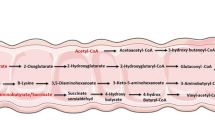
SCFAs are absorbed by colonocytes, mainly through the hydrogen and sodium dependent monocarboxylate transporters (MCTs and SMCTs) and by passive diffusion [45]. Tissues have different subtypes and patterns of MCT expression—proton-coupled monocarboxylate transporter 1 (MCT1/SLC16A1) and sodium-coupled monocarboxylate transporter (SMCT1/SLC5A8) [46]. SCFAs are rapidly absorbed by colonocytes via MCT1 and SMCT1, passively diffused or exchanged with bicarbonate (HCO3−) via exchangers of unknown identity, and then partially oxidized to CO2, producing energy for the cell in the form of ATP. CD147 is the chaperone (ancillary protein) for MCT1 [46]. Meanwhile, SCFAs can engage G-protein-coupled receptors (GPCRs) on the surface of cells regulating intracellular signaling pathways [13]. Acetate is produced from pyruvate via acetyl-CoA and it is also used to produce butyrate via Butyryl-CoA [47]. Propionate is produced from phosphoenolpyruvate via the acrylate and succinate pathways [23]. These SCFAs participate in the tricarboxylic acid cycle(TCA cycle) and generate ATP in the mitochondria of intestinal epithelial cells (Fig. 1) [48]. Intracellular actions of SCFA in colonic epithelium involve inhibition of histone deacetylases (HDACs), generation of energy, and conversion into ketone bodies [46]. Clostridium butyricum, one of the most commonly observed SCFAs producing probiotics, can inhibit the Wnt/β-catenin signaling pathway by inhibiting HDACs activity, and thus prevent the development of intestinal tumors in a murine model [13]. HDACs activity also be inhibited by butyrate to induces G1 cell cycle arrest and differentiation of human colon carcinoma cells by upregulating the negative cell cycle regulator p21Waf1/Cip1 [49].
Mechanisms of SCFA action within intestinal epithelial cells. SCFA short-chain fatty acids, MCT1 monocarboxylate transporter 1, SMCT1 sodium-coupled monocarboxylate transporter 1, ACSS1 acyl-CoA synthetase short-chain family member 1, ACSS2 acyl-CoA synthetase short-chain family member 2, ATP Adenosine triphosphate
SCFAs that are not metabolized by colonocytes enter the portal circulation of the liver through the basolateral membrane and provide energy substrates for hepatocytes through oxidation [50]. Only a small amount of acetate, propionate and butyrate reach the systemic circulation and other tissues such as skeletal muscle and adipose tissue. Recent studies on SCFAs have used fecal determination to reflect colon production of SCFAs [51,52,53]. In vitro experiments, Zuo et al. [54] found butyrate suppresses proliferation and migration of RKO colon cancer cells though regulating endocan expression by MAPK signaling pathway. In short, SCFAs plays a huge role in preventing the occurrence and development of CRC.
The molecular mechanism of SCFAs in CRC progression is a complex process. The gut microbiome plays a crucial role in the development of CRC by disrupting the homeostasis of the microenvironment and altering immune responses. Dysfunction of the gut microbiota can promote the occurrence of colorectal cancer, and SCFAs, as metabolites of the gut microbiota, may play a key role in this process. As an energy substrate for colon cells, SCFAs have anti-inflammatory and anticancer properties [6]. In patients with colitis, butyrate induces the release of IL-18 from colon epithelial cells by activating GPR109A, thereby participating in the regulation of colitis and colon cancer [55]. SCFAs also protect intestinal health by inducing autophagy in colon cancer cell lines [37, 56].
T cells play a crucial role in maintaining a stable intestinal environment, and SCFAs directly or indirectly regulate T cell differentiation and participate in specific cellular immunity [57]. SCFAs can alleviate intestinal inflammation by inhibiting HDACs and regulating the mTOR S6K pathway to induce the production of effector T cells and regulatory T cells [58]. So far, butyrate has been shown to prevent colitis and colon cancer under low fiber diet conditions, affecting the function of colorectal cancer cells, including regulating gene expression [59], cell signal transduction [54], and inhibiting the growth of colon cancer cells [28, 35]. The molecular mechanisms underlying the effects of propionate and acetate on colorectal cancer are also summarized in Table 1.
4 Dietary fiber and SCFAs supplementation
Most nutritionists and physicians believe that a balanced diet can maximize the resistance and prevention of digestive diseases and malignant tumors. Nowadays, many young people have an imbalanced high-fat, high-meat, low-fibre diet, the proinflammatory and proneoplastic properties of protein fermentation and bile acid deconjugated residues predominate, leading to increased colon cancer risk, so colorectal cancer has a younger trend [16]. High-fibre diet is thought to provide a variety of health benefits. In addition to increasing the speed of fecal bulking and transport along the colon, fiber also provides a wide range of phytochemicals and metabolites transformed by bacteria in the human colon, of which the most important fermentation product is SCFA. A review showed high-fibre diet, in particular cereal fibre and whole grains, associated with approximately 10% lowered risk of developing CRC [12]. A prospective cohort study also demonstrated increasing fiber consumption after CRC diagnosis has been associated with better survival rates [60].
In order to provide sufficient SCFAs to the body, according to the "Chinese Residents' Dietary Nutrient Reference Intakes (2021 Edition)", adults should consume more than 25 g of dietary fiber per day. European Food Safety Authority adviced a daily intake of fiber between 25 and 32 g/d for adult women and 30–35 g/d for adult men, and for children and older adults 3–4 g/d approximately [61]. There are now two ways to consume dietary fiber. First of all, through dietary intake, such as peas, sugar beets, chicory, garlic asparagus, banana, corn, wheat, tapioca cereals, etc., but it is actually difficult to meet the demand. The second is choose products containing dietary fiber directly, such as inulin, polysaccharide, resistant dextrin or starch, etc. In fact, while engaging in dietary fiber intake, a low-fat diet is also necessary. Bile acid (BA) concentrations can reach 1 mM in the colon after the consumption of a high-fat meal, and these BAs, mostly secondary BAs in humans, are believed to be promoters of colon cancer [62]. The importance of a balanced diet deserves our attention.
5 Conclusion
Although studies on the effects of SCFAs seem to show that supplements have generally positive effects on CRC, in order to obtain maximum efficacy, efforts should be made to carry out high-quality randomized controlled trials to determine the mechanism of action, the best timing, dosage, source, extraction, preparation and quantification of these products, as well as very suitable nutrition questionnaires. This will enable us to set the use of these compounds in clinical guidelines for cancer prevention.
Data availability
No datasets were generated or analysed during the current study.
References
Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc. 2015;74(1):13–22.
Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL Jr. Short chain fatty acids and their receptors: new metabolic targets. Transl Res. 2013;161(3):131–40.
Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41.
Gomes SD, Oliveira CS, Azevedo-Silva J, Casanova MR, Barreto J, Pereira H, et al. The role of diet related short-chain fatty acids in colorectal cancer metabolism and survival: prevention and therapeutic implications. Curr Med Chem. 2020;27(24):4087–108.
McNabney SM, Henagan TM. Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer obesity and insulin resistance. Nutrients. 2017;9(12):1348.
Yao Y, Cai X, Fei W, Ye Y, Zhao M, Zheng C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. 2022;62(1):1–12.
He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21(17):6356.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pract. 2012;27(5):613–23.
Domingo JL, Nadal M. Carcinogenicity of consumption of red meat and processed meat: A review of scientific news since the IARC decision. Food Chem Toxicol. 2017;105:256–61.
Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–600.
Hou H, Chen D, Zhang K, Zhang W, Liu T, Wang S, et al. Gut microbiota-derived short-chain fatty acids and colorectal cancer: ready for clinical translation? Cancer Lett. 2022;526:225–35.
Ocvirk S, Wilson AS, Posma JM, Li JV, Koller KR, Day GM, et al. A prospective cohort analysis of gut microbial co-metabolism in Alaska Native and rural African people at high and low risk of colorectal cancer. Am J Clin Nutr. 2020;111(2):406–19.
Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343: d6617.
Chattopadhyay I, Dhar R, Pethusamy K, Seethy A, Srivastava T, Sah R, et al. Exploring the role of gut microbiome in colon cancer. Appl Biochem Biotechnol. 2021;193(6):1780–99.
O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691–706.
Lewis K, Lutgendorff F, Phan V, Soderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis. 2010;16(7):1138–48.
Dabek-Drobny A, Kaczmarczyk O, Wozniakiewicz M, Pasko P, Dobrowolska-Iwanek J, Wozniakiewicz A, et al. Association between fecal short-chain fatty acid levels, diet, and body mass index in patients with inflammatory bowel disease. Biology. 2022;11(1):108.
Kaczmarczyk O, Dabek-Drobny A, Wozniakiewicz M, Pasko P, Dobrowolska-Iwanek J, Wozniakiewicz A, et al. Fecal levels of lactic, succinic and short-chain fatty acids in patients with ulcerative colitis and crohn disease: a pilot study. J Clin Med. 2021;10(20):4701.
Zheng DW, Li RQ, An JX, Xie TQ, Han ZY, Xu R, et al. Prebiotics-encapsulated probiotic spores regulate gut microbiota and suppress colon cancer. Adv Mater. 2020;32(45): e2004529.
Sanchez-Alcoholado L, Ramos-Molina B, Otero A, Laborda-Illanes A, Ordonez R, Medina JA, et al. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers. 2020;12(6):1406.
Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci. 2019;20(5):1214.
Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–72.
Saffarian A, Mulet C, Regnault B, Amiot A, Tran-Van-Nhieu J, Ravel J, et al. Crypt- and mucosa-associated core microbiotas in humans and their alteration in colon cancer patients. mBio. 2019. https://doi.org/10.1128/mBio.01315-19.
Wu N, Yang X, Zhang R, Li J, Xiao X, Hu Y, et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb Ecol. 2013;66(2):462–70.
Campos-Perez W, Martinez-Lopez E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(5): 158900.
Chapkin RS, Navarro SL, Hullar MAJ, Lampe JW. Diet and gut microbes act coordinately to enhance programmed cell death and reduce colorectal cancer risk. Dig Dis Sci. 2020;65(3):840–51.
Ma X, Zhou Z, Zhang X, Fan M, Hong Y, Feng Y, et al. Sodium butyrate modulates gut microbiota and immune response in colorectal cancer liver metastatic mice. Cell Biol Toxicol. 2020;36(5):509–15.
Cho Y, Turner ND, Davidson LA, Chapkin RS, Carroll RJ, Lupton JR. Colon cancer cell apoptosis is induced by combined exposure to the n-3 fatty acid docosahexaenoic acid and butyrate through promoter methylation. Exp Biol Med. 2014;239(3):302–10.
Hong MY, Turner ND, Murphy ME, Carroll RJ, Chapkin RS, Lupton JR. In vivo regulation of colonic cell proliferation, differentiation, apoptosis, and P27Kip1 by dietary fish oil and butyrate in rats. Cancer Prev Res. 2015;8(11):1076–83.
Matthews GM, Howarth GS, Butler RN. Short-chain fatty acids induce apoptosis in colon cancer cells associated with changes to intracellular redox state and glucose metabolism. Chemotherapy. 2012;58(2):102–9.
Ali SR, Orang A, Marri S, McKinnon RA, Meech R, Michael MZ. Integrative transcriptomic network analysis of butyrate treated colorectal cancer cells. Cancers. 2021. https://doi.org/10.3390/cancers13040636.
Klepinina L, Klepinin A, Truu L, Chekulayev V, Vija H, Kuus K, et al. Colon cancer cell differentiation by sodium butyrate modulates metabolic plasticity of Caco-2 cells via alteration of phosphotransfer network. PLoS ONE. 2021;16(1): e0245348.
Hu S, Liu L, Chang EB, Wang JY, Raufman JP. Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced miR-17-92a cluster transcription in human colon cancer cells. Mol Cancer. 2015;14:180.
Broadfield LA, Saigal A, Szamosi JC, Hammill JA, Bezverbnaya K, Wang D, et al. Metformin-induced reductions in tumor growth involves modulation of the gut microbiome. Mol Metab. 2022;61: 101498.
Dong J, Wang B, Xiao Y, Liu J, Wang Q, Xiao H, et al. Roseburia intestinalis sensitizes colorectal cancer to radiotherapy through the butyrate/OR51E1/RALB axis. Cell Rep. 2024;43(3): 113846.
Tang Y, Chen Y, Jiang H, Nie D. Short-chain fatty acids induced autophagy serves as an adaptive strategy for retarding mitochondria-mediated apoptotic cell death. Cell Death Differ. 2011;18(4):602–18.
Ryu TY, Kim K, Han TS, Lee MO, Lee J, Choi J, et al. Human gut-microbiome-derived propionate coordinates proteasomal degradation via HECTD2 upregulation to target EHMT2 in colorectal cancer. ISME J. 2022;16(5):1205–21.
Casanova MR, Azevedo-Silva J, Rodrigues LR, Preto A. Colorectal cancer cells increase the production of short chain fatty acids by propionibacterium freudenreichii impacting on cancer cells survival. Front Nutr. 2018;5:44.
Ryu TY, Kim K, Son MY, Min JK, Kim J, Han TS, et al. Downregulation of PRMT1, a histone arginine methyltransferase, by sodium propionate induces cell apoptosis in colon cancer. Oncol Rep. 2019;41(3):1691–9.
Marques C, Oliveira CS, Alves S, Chaves SR, Coutinho OP, Corte-Real M, et al. Acetate-induced apoptosis in colorectal carcinoma cells involves lysosomal membrane permeabilization and cathepsin D release. Cell Death Dis. 2013;4(2): e507.
Oliveira CS, Pereira H, Alves S, Castro L, Baltazar F, Chaves SR, et al. Cathepsin D protects colorectal cancer cells from acetate-induced apoptosis through autophagy-independent degradation of damaged mitochondria. Cell Death Dis. 2015;6(6): e1788.
Rodríguez-Enríquez S, Robledo-Cadena DX, Gallardo-Pérez JC, Pacheco-Velázquez SC, Vázquez C, Saavedra E, et al. Acetate promotes a differential energy metabolic response in human HCT 116 and COLO 205 colon cancer cells impacting cancer cell growth and invasiveness. Front Oncol. 2021;11: 697408.
Garcia JA, Chen R, Xu M, Comerford SA, Hammer RE, Melton SD, et al. Acss2/HIF-2 signaling facilitates colon cancer growth and metastasis. PLoS ONE. 2023;18(3): e0282223.
Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. 2014;20(10):1487–98.
Sivaprakasam S, Bhutia YD, Yang S, Ganapathy V. Short-chain fatty acid transporters: role in colonic homeostasis. Compr Physiol. 2017;8(1):299–314.
Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–45.
Liu P, Wang Y, Yang G, Zhang Q, Meng L, Xin Y, et al. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res. 2021;165: 105420.
Sanaei M, Kavoosi F. Effect of 5-Aza-2’-deoxycytidine in comparison to valproic acid and trichostatin a on histone deacetylase 1, DNA methyltransferase 1, and CIP/KIP Family (p21, p27, and p57) genes expression, cell growth inhibition, and apoptosis induction in colon cancer SW480 cell line. Adv Biomed Res. 2019;8:52.
Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57(6):943–54.
Skonieczna-Zydecka K, Grochans E, Maciejewska D, Szkup M, Schneider-Matyka D, Jurczak A, et al. Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients. 2018;10(12):1939.
Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–55.
Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915–32.
Zuo L, Lu M, Zhou Q, Wei W, Wang Y. Butyrate suppresses proliferation and migration of RKO colon cancer cells though regulating endocan expression by MAPK signaling pathway. Food Chem Toxicol. 2013;62:892–900.
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–39.
Zhang J, Yi M, Zha L, Chen S, Li Z, Li C, et al. Sodium butyrate induces endoplasmic reticulum stress and autophagy in colorectal cells: implications for apoptosis. PLoS ONE. 2016;11(1): e0147218.
Akhtar M, Chen Y, Ma Z, Zhang X, Shi D, Khan JA, et al. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim Nutr. 2022;8:350–60.
Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8(1):80–93.
Kurata N, Tokashiki N, Fukushima K, Misao T, Hasuoka N, Kitagawa K, et al. Short chain fatty acid butyrate uptake reduces expressions of prostanoid EP(4) receptors and their mediation of cyclooxygenase-2 induction in HCA-7 human colon cancer cells. Eur J Pharmacol. 2019;853:308–15.
Song M, Wu K, Meyerhardt JA, Ogino S, Wang M, Fuchs CS, et al. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol. 2018;4(1):71–9.
Stephen AM, Champ MM, Cloran SJ, Fleith M, van Lieshout L, Mejborn H, et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. 2017;30(2):149–90.
Farhana L, Nangia-Makker P, Arbit E, Shango K, Sarkar S, Mahmud H, et al. Bile acid: a potential inducer of colon cancer stem cells. Stem Cell Res Ther. 2016;7(1):181.
Funding
Shenyang Science and Technology Bureau (20-205-4-096).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, G., Tang, J., Zhou, J. et al. Short-chain fatty acids play a positive role in colorectal cancer. Discov Onc 15, 425 (2024). https://doi.org/10.1007/s12672-024-01313-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01313-5