Abstract
Background
Chemotherapy is the main treatment strategy for patients with advanced HER2-negative gastric cancer (GC); yet, many patients do not respond well to treatment. This study evaluated the sensitivity of a mini patient-derived xenograft (MiniPDX) animal model in patients with HER2-negative intermediate-advanced GC.
Methods
In this single-arm, open-label clinical study, we consecutively recruited patients with HER2-negative advanced or recurrent GC from September 2018 to July 2021. Tumor tissues were subjected to MiniPDX drug sensitivity tests for screening individualized anti-tumor drugs; appropriate drug types or combinations were selected based on drug screening results. The primary endpoints were progression-free survival (PFS) and safety, and the secondary endpoints were overall survival (OS) and objective response rate (ORR).
Results
A total of 17 patients were screened, and 14 eligible patients were included.The median follow-up time was 9 (2–34) months. The median PFS time was 14.1 (2–34) months, the median OS time was 16.9 (2–34) months, ORR was 42.9% (6/14), and DCR was 92.9% (13/14). The most common treatment-related adverse events (TRAE) were fatigue (14 (100%)), anorexia (13 (93%)) and insomnia (12 (86%)), and the most common grade 3 or worse TRAE was fatigue (6 (43%)), and anorexia (6 (43%)). The occurrence rate of myelosuppression, nausea and vomiting, abnormal liver enzymes, and other grade 3–4 chemotherapy adverse reactions were relatively low, and no grade 5 treatment-related adverse events occurred.
Conclusion
Screening HER2-negative medium-advanced GC/GJC chemotherapy regimens and targeted drugs based on MiniPDX animal models showed good tumor activity and safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to the latest global cancer burden data recently released by the International Agency for Research on Cancer (IARC) [1], 1.1 million people were diagnosed with gastric cancer (GC) in 2020. Gastric cancer has the highest morbidity and mortality rate among malignant digestive system tumors. Most cases are diagnosed with an advanced stage. Among advanced-stage GC patients who receive surgery, 60% experience local recurrence or distant metastasis. Human epidermal growth factor receptor 2 (HER2) is a member of the receptor family related to tumor cell proliferation, apoptosis, adhesion, migration and differentiation, and is involved in the pathogenesis and adverse results of a variety of cancers, including advanced gastric cancer (AGC) and gastroesophageal junction cancer (GJC) [2, 3]. The positive rate of HER2 in GC was about 12% ~ 20% [4]. Trastuzumab is a monoclonal antibody targeting HER2, which exerts antitumor effects by inducing antiantibody dependent cytotoxicity and inhibiting HER2-mediated signalling [5]. The phase 3 ToGA study demonstrated the clinical benefits of trastuzumab combined with chemotherapy in patients with previously untreated, unresectable or metastatic HER2-positive (HER2 +) GC or GEJ cancer [6], and HER2-targeted combined chemotherapy has become the first-line treatment for HER2-positive advanced GC [7, 8]. Yet, there are no standard first-line treatments for advanced or recurrent gastric cancer with HER2 negative. Immunotherapy combined with chemotherapy is a common treatment approach; still, a high number of patients do not respond well to treatment. Monotherapy is the most common second- and third-line treatment [9, 10]. Accordingly, Precision treatment is particularly important for patients with HER2-negative advanced or recurrent gastric cancer.
The patient-derived xenograft (PDX) model has come into our view, which is commonly used for in vivo experiments. Compared to animal models established using immortalized cell lines, the use of patient tissue can more realistically reflect the molecular diversity and cellular and histological heterogeneity of tumors in patients. PDX model has been widely used for drug evaluation, biomarker identification, biological research, and personalized medicine strategies [11,12,13]. However, the establishment of PDX may require a longer time (4 to 8 months) compared to other models, which is also a major limitation considering that most patients with advanced-stage have a poor prognosis. Thus, a MiniPDX model has been proposed. MiniPDX model is established by injecting patient-derived tumor cells into hollow fiber capsules, which significantly saves time [14]. So far, this model has been applied to a variety of solid tumors [15].
The application of miniPDX model in the treatment of GC has also been reported [15]. Zhu et al. [16] found that drugs screened by OncoVeeTM-Mini-PDX have significant benefits for a patient with HER2-positive advanced gastric cancer. Moreover, Wang et al. [17] successfully established a MiniPDX model for four patients (three patients were HER2 positive, and one patient was HER2 negative), where two patients achieved partial remission after treatment, while the disease progressed in the other two patients, and no serious adverse reactions were observed. In addition, Ge et al. found that MiniPDX can prolong the survival of patients with HER2-negative gastric cancer with liver metastases (GCLM) and improve efficacy [18]. In this study, we established an individualized drug screening system through the MiniPDX model, and used a prospective single-arm open-label trial aiming to prolong the survival of HER2-negative GC patients.
2 Materials and methods
2.1 Selection criteria of patients
This single-arm clinical study (registration number: ChiCTR1800019568; registration institution: Chinese Clinical Trial Registry) evaluated the safety and antitumor activity of an individualized drug screening protocol established by the MiniPDX model in patients with advanced GC/GJC.
Inclusion criteria:1.GC/GJC patients aged between 18 and 80 who cannot undergo surgery.2.It is a stage III/IV intermediate-advanced malignancy confirmed by imaging or histology, and is negative for Her-2 as confirmed by FISH.3.The time from the start of the last chemotherapy is ≥ 4 weeks.4.Fresh samples of tumor tissue can be obtained by surgical excision or biopsy 5. At least one objective tumor that meets the RECIST 1.1 standard can be evaluated using CT/MRI for efficacy 6. The ECOG score is ≤ 2 points, and the expected survival period must be ≥ 3 months.
Exclusion criteria: 1. Patients who cannot obtain sufficient tumor cells.2. Patients who cannot receive follow-up or are participating in other clinical trials. 3. Patients with severe cardiovascular and cerebrovascular diseases and severe abnormalities in liver and kidney functions. 4. Patients with multiple cancers.
Exclusion criteria: 1. Subject withdraws informed consent 2. Those who have lost follow-up within half a year after enrollment and have not been followed up as required. 3. The researcher believes that the subject is not suitable for continuing to participate in the trial.
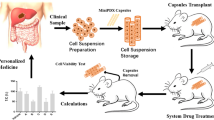
2.2 OncoVeeM-MiniPDX model establishment and drug sensitivity test
The MiniPDX model was established by taking the primary tumor specimens resected by gastroscopic biopsy or puncture or palliative surgery. Drug susceptibility was detected by the OncoVee™ MiniPDX method (Shanghai LIDE Biotech Co., Ltd.)[14]. GC tissue samples were washed with Hank’s balanced salt solution (HBSS) to remove non-tumor tissues, such as blood vessels and necrotic tumor tissues. After sectioning, the tumor tissues were digested with collagenase at 37 °C for 1–2 h, the cells were collected, and blood cells and fibroblasts were removed. The GC cell suspension was then transferred to HBSS rinsed capsules.
BALB/c nude mice with 4-week-old (SLARC Inc., Shanghai, China) were selected as animal models. A total of 4–6 capsules were implanted subcutaneously on their backs. One day after inoculation of tumor cells, according to the treatment plan given by the researcher, appropriate drugs or their combinations (Table 1) were given to mice for 7 days, and nude mice treated with normal saline were set up as controls. After 7 days, the cells in the capsules were taken out, and the relative fluorescence units (RFU) of tumor cells were measured by CellTiter-Glo fluorescence cell viability assay. The relative proliferation rate of tumor cells was calculated (T/C%) (T/C% = (RFU of drug treatment group on day 7 − RFU of drug treatment group on day 0) / (RFU of the control group on day 7 − RFU of the control group on day 0) × 100%)). Each experiment was conducted six times and the average value was reported. When the tumor growth inhibition rate was > 50% compared with the blank group, the regimen was defined “sensitive” [20].
The chemotherapy regimen with the highest chemosensitivity was given to the patients corresponding to the tumor cells for chemotherapy, and the drug was used until disease progression, death, intolerance of adverse reactions, withdrawal of consent, investigator’s decision, or completion of the 24-month study.
2.3 Results and measurements
The primary endpoints of this study were progression-free survival (PFS) and safety. Secondary endpoints were overall survival (OS), objective response rate (ORR), and disease control rate (DCR), all determined according to RECIST 1.1. PFS was defined as the time from the first drug administration to the first documented disease progression or death from any cause, whichever occurred first. OS was defined as the time from first drug administration to death from any cause. Patients were divided into four subgroups: complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). ORR was defined as the percentage of CR and PR patients among all patients. DCR was defined as the percentage of patients achieving CR, PR, and SD.
All patients underwent routine CT scanning of the upper abdomen during baseline and follow-up using a Somatom PLUS-S CT scanner (Siemens Medical Systems, Erlangen, Germany). CT images were processed using the 3D slice software package (version 4.7). At least two radiologists with 10 + years of work experience and a research assistant completed the process. During treatment, radiographic assessments of short-term efficacy were performed every two cycles until disease progression or death, according to RECIST 1.1. Adverse events were monitored and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0), and all adverse events were reported from the time of treatment assignment to 90 days after treatment cessation.
2.4 Follow up
Follow-up was performed every 6 weeks during treatment and every 3 months after treatment until death. Follow-up evaluation included medical history, physical examination, blood routine, liver and kidney function, and related tumor markers, with telephone follow-up for those who were not regularly reviewed.During each follow-up, patients’ quality of life was quantitatively measured by the QLQ-C30 scale, from overall health, functional dimensions (including physical function, role function, emotional function, cognitive function, and social function), and symptom-related dimensions (including fatigue, nausea, and vomiting, pain, dyspnea, insomnia, poor appetite, constipation, diarrhea, etc.) to evaluate and calculate the standard score (SS) of the overall health status: SS = [(RS-1)/6] *100, RS = (Overall health status score + Overall quality of life score)/2.
2.5 Statistical analysis
Microsoft Excel 2019was used to establish a database, SPSS 22.0 was used for statistical analysis, and GraphPad Prism 8.0was used for plotting figures. The Kolmogorov-Smirnov (K-S) method and the Shapiro–Wilk (S-W) method were applied for the normality test. The age distribution of the patients conformed to the normal distribution and was expressed asX ± SD. The PFS and OS curves were calculated by the Kaplan-Meier method. The overall quality of life scores of patients before and after treatment were compared using paired T-test. P < 0.05 was considered to be statistically significant.
2.6 Ethical approval
This study was approved by the Ethics Committee of Wuxi Hospital Affiliated to Nanjing University of Chinese Medicine (201809001J01-01). The animal study was reviewed and approved by the Institutional Animal care and use Committee(IACUC) Shanghai LIDI Biotech,Co.LTD (LDIACUC001). This research study was conducted following the Declaration of Helsinki, and all patients signed informed consent.All institutional and national guidelines for the care and use of laboratory animals were followed.
3 Results
3.1 General information of patients
Between September 2018 and July 2021, 17 patients were screened; 2 patients did not meet the inclusion criteria and 1 patient met the exclusion criteria (Fig. 1). The MiniPDX model was successfully established in the remaining 14 patients, and at least one course of drug susceptibility testing was included in the primary analysis. The mean age of the 14 patients was 71.57 ± 9.78 years. Eleven (79%) patients had GC; 3 (21%) patients had GJC. In terms of clinical-stage, 10 (71%) patients were at stage IV, 3 (21%) patients were at stage IIIC, and 1 (7.14%) was at stage IIIB. In addition, 4 (29%) patients had one distant metastasis, 5 (36%) patients had multiple distant metastases, and the others had no distant metastases.Five patients had no previous treatment, seven patients had first-line treatment, and two patients had second-line or third-line treatment. (Table 2). The median follow-up time for data analysis (data cutoff was December 31, 2021) was 9.0 (2–34) months. The most common reason for discontinuation of treatment was disease progression in 7 (50%) patients and adverse events in 2 (14%) patients (Fig. 1).
3.2 Results of MiniPDX model detection
The intraoperative tumor tissues of 14 patients in the drug-susceptibility group were submitted for examination, and a MiniPDX model was established. According to the pathological type of the patient’s tumor and the basic condition of the patient, the researchers proposed 3 treatment regimens in combination with the NCCN guidelines (2016 edition) [19], which were then applied to the mice for drug susceptibility testing. The results showed that an average of 0.5 (± 0.31) different types of regimens showed a satisfactory inhibitory effect (T/C% < 50%) for each patient (Table 3).
3.3 Evaluation of short-term clinical efficacy of patients
According to the drug susceptibility results, a chemotherapy regimen with high sensitivity (i.e., with a low proliferation rate) was selected. The final chemotherapy protocol and corresponding efficacy evaluation showed that among the 14 patients, 3 were CR, 3 were PR, 7 were SD, and 1 was PD (Table 4). The short-term efficacy evaluation within 24 weeks showed 2 were CR, 3 were PR, 8 were SD, and 1 was PD (Table 5). The final ORR was 42.9% (6/14, 95% CI 17.7–71.1%) and DCR was 92.9% (13/14, 95% CI 66.1–99.8%) (Table 6). DOR occurred in patient 5, 6, and 7 at 4 months, 6 months, and 13 months, respectively. Treatment was discontinued in 3 (21.4%) patients due to disease progression and in 6 (42.9%) patients due to death, with a mean duration of 6.6 ± 4.1 months. The swimming plot and waterfall plot are shown in Fig. 2.
3.4 Evaluation of patients’ survival
As of the data cutoff time, 6 cases of the 14 patients achieved the final outcome OS, 6 cases were alive without progression, and 2 cases died of other causes, including 1 case due to a car accident and 1 case due to a cerebrovascular accident. The median progression-free survival (mPFS) was 14.1 months (95% confidence interval: 1.18–14.82, Fig. 3A), and the mOS was 16.9 months (95% confidence interval: 2.49–23.51, Fig. 3B).
3.5 Evaluation of patients’ quality of life
The overall quality of life score of 14 patients slightly decreased after treatment compared with that before treatment; the median at baseline was 75 (50–83.33) points while the median after treatment was 66.67 (25–83.33) points (P = 0.018) (Fig. 4)(Supplementary Table S1).
3.6 Adverse reactions of patients
Fourteen patients had different degrees of adverse reactions during treatment (Table 7), most of which were low-grade TRAEs, without grade 5 adverse reactions, and TRAEs were controllable. Generally, there were no new safety signals and no signs of synergistic toxicity.
4 Discussion
Cancer is a highly heterogeneous disease affected by genomic, epigenetic, and transcriptome changes in cells. Even tumors originating from the same organ have differences, so personalized medicine or personalized care is crucial when designing the treatment plan [21]. Over the years, personalized therapies based on genetic test results of GC/GJC patients have been increasing. In this study, three different combined chemotherapy regimens were selected for different GC/GJC patients according to NCCN guidelines (2016 edition) [19]. The sensitivity of these three combined chemotherapy regimens was analyzed using a MiniPDX model, and the chemotherapy regimen with the lowest proliferation rate was selected for individualized treatment of patients according to the detection results. The results showed that ORR was 42.86% (6/14) and DCR was 92.86% (13/14); the mean PFS time was 14.1 months (95% CI 1.18 -14.82), and the mean OS time was 16.9 months (95% CI 2.49–23.51). The overall quality of life score was slightly lower after treatment than before treatment, and the occurrence rate of grade 3–4 chemotherapy adverse reactions such as bone marrow suppression, nausea and vomiting, and abnormal liver enzymes was relatively low. Therefore, the MiniPDX-guided treatment was found to have better safety and better survival time.
Fluorouracil plus platinum-based chemotherapy is the most commonly used first-line therapy for unresectable advanced or metastatic human epidermal growth factor receptor 2 (HER2)-negative gastric and gastroesophageal junction adenocarcinoma, with a median overall survival rate (OS) less than 1 year [22,23,24]. However, the clinical benefit rates of second-line chemotherapy, including SPA (S-1 and paclitaxel) [24], XELOX (capecitabine and oxaliplatin) [25, 26], DOCOX (docetaxel plus oxaliplatin) [27], S-1 monotherapy, XELIRI (capecitabine and irinotecan) [28] and some newly developed targeted agents (such as apatinib monotherapy) [29], were even more limited. At present, no large randomized controlled clinical trial has been able to prove that maintenance chemotherapy can improve patient survival time. Although new targeted therapies provide more possibilities for maintenance therapy strategies, most completed clinical trials failed to achieve OS prolongation [8].In our study, one patient was reported to have an EGFR mutation by genetic testing, and this overexpression was associated with poor prognosis [30]. The combination of anti-EGFR therapy with chemotherapy provides a basis for the treatment of resistant GC patients.Several phase II clinical trials demonstrated the benefit of combination chemotherapy and anti-EGFR therapy in GC patients [31,32,33], so we tried drug susceptibility testing with AZD9291, but the results were not satisfactory.
The patient-derived xenograft (PDX) model is commonly used for in vivo experiments, as it can reflect the molecular diversity and cellular and histological heterogeneity of tumors in patients [34,35,36]. However, the long trial period and unsatisfactory implantation rate hinder the widespread application of PDX in some advanced malignancies, especially in GC [37]. In recent years, the clinical application of MiniPDX has become more and more common, and encouraging positive results have been obtained in the application of hepatocellular carcinoma [38] and gallbladder carcinoma [39]. There were also different potential applications in other solid tumors. For example, in another case reported by Zhao et al. [40], personalized treatment based on MiniPDX and whole-exome sequencing was used to rapidly assess drug sensitivity and reveal significant genetic changes in patients with metastatic duodenal adenocarcinoma. Moreover, Liu et al. [41] found that gemcitabine and XCT790 have synergistic antitumor effects on pancreatic cancer by the MiniPDX model. By establishing the MiniPDX model, Xu et al. [42] found that the combined application of AKT inhibitors and PARP inhibitors might be a feasible method for clinical trials in patients with recurrent ovarian cancer. As mentioned above, the research of MiniPDX in advanced gastric cancer is still limited to retrospective studies and case reports. Our prospective study revealed a longer median PFS time and median OS time, thus providing more convincing evidence for the feasibility of MiniPDX in HER2-negative advanced gastric cancer.
However, the drug susceptibility testing technology of MiniPDX also has certain limitations. First, the effects of chemotherapy, which worked by regulating the cell cycle, might not be adequately reproduced because the MiniPDX model was used within 7 days, so the MiniPDX model could not simulate the actual administration and effects of the entire cycle. Second, based on the Checkmate649 study [43], immunotherapy had been included in the first-line treatment of advanced gastric cancer. Since our study was carried out in 2018 and immunotherapy was not included in the latest guidelines at that time, the PD-L1 expression rate of patients was not detected, and immune drugs were not included in the drug sensitivity test. Moreover, the nude mice used in this study could not reproduce the human immune environment, meaning that the efficacy of combined immunotherapy could not be tested. Yet, a humanized peripheral blood mononuclear cell (PBMC) reconstruction model of immunodeficient mice could be used to evaluate the efficacy of immunotherapy drugs, and drug sensitivity testing programs can be extended to guide clinical medication in the future. Other shortcomings are a lack of multi-center, multi-sample size, and control group. In the future, we plan to further expand the sample size and summarize more clinical data to obtain a higher-level evidence-based basis.
In conclusion, the MiniPDX model has shown to be an effective tool to guide the choice of drug regimens for GC/GJC patients, providing a scientific basis for clinical conversion therapy of GC/GJC, reducing adverse reactions to clinical experience-guided medication, and revealing broad application prospects. In the future, randomized controlled trials with larger sample sizes are needed to further clarify the efficacy and safety.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a J Clin. 2021;71(3):209–49.
Boku N. HER2-positive gastric cancer. Gastric cancer. 2014;17(1):1–12.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann oncol. 2008;19(9):1523–9.
Van Cutsem E, Bang Y, Feng-Yi F, Xu J, Lee K, Jiao S, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric cancer. 2015;18(3):476–84.
Hudis C. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51.
Bang Y, Van Cutsem E, Feyereislova A, Chung H, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London, England). 2010;376(9742):687–97.
Bang K, Cheon J, Park Y, Kim H, Ryu M, Park Y, et al. Association between HER2 heterogeneity and clinical outcomes of HER2-positive gastric cancer patients treated with trastuzumab. Gastric cancer. 2022;25(4):794–803.
Yao Y, Deng R, Liao D, Xie H, Zuo J, Jia Y, et al. Maintenance treatment in advanced HER2-negative gastric cancer. Clin Transl Oncol. 2020;22(12):2206–12.
Wang F, Zhang X, Li Y, Tang L, Qu X, Ying J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (London, England). 2021;41(8):747–95.
Ajani J, D’Amico T, Bentrem D, Chao J, Corvera C, Das P, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN clinical practice guidelines in oncology. J Nat Compr Cancer Netw. 2019;17(7):855–83.
Okada S, Vaeteewoottacharn K, Kariya R. Application of highly immunocompromised mice for the establishment of patient-derived xenograft (PDX) models. Cells. 2019. https://doi.org/10.3390/cells8080889.
Yoshida G. Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 2020;13(1):4.
Rivera M, Fichtner I, Wulf-Goldenberg A, Sers C, Merk J, Patone G, et al. Patient-derived xenograft (PDX) models of colorectal carcinoma (CRC) as a platform for chemosensitivity and biomarker analysis in personalized medicine. Neoplasia (New York, NY). 2021;23(1):21–35.
Zhang F, Wang W, Long Y, Liu H, Cheng J, Guo L, et al. Characterization of drug responses of mini patient-derived xenografts in mice for predicting cancer patient clinical therapeutic response. Cancer Commun (London, England). 2018;38(1):60.
Huang Y, Xu J, Li K, Wang J, Dai Y, Kang Y. A novel, personalized drug-screening system for platinum-resistant ovarian cancer patients: a preliminary clinical report. Cancer Manag Res. 2021;13:2849–67.
Zhu X, Zhu Y, Chen N, Tang C, Shi J. The drugs screened by OncoVee-Mini-PDX have significantly benefited the patient with HER2-positive advanced gastric cancer. J Oncol Pharm Pract. 2022;28(6):1435–40.
Wang J, Huang J, Wang H, Yang W, Bai Q, Yao Z, et al. Personalized treatment of advanced gastric cancer guided by the MiniPDX model. J Oncol. 2022;2022:1987705.
Ge Y, Zhang X, Liang W, Tang C, Gu D, Shi J, et al. OncoVee™-MiniPDX-guided anticancer treatment for gastric cancer patients with synchronous liver metastases: a retrospective cohort analysis. Front Oncol. 2021;11: 757383.
Ajani J, D’Amico T, Almhanna K, Bentrem D, Chao J, Das P, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Network. 2016;14(10):1286–312.
Li C, Sun Y, Yu G, Cui J, Lou Z, Zhang H, et al. Integrated Omics of Metastatic Colorectal Cancer. Cancer Cell. 2020;38(5):734-47.e9.
Cho S. Patient-derived xenografts as compatible models for precision oncology. Laboratory animal research. 2020;36:14.
Fuchs C, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran S, Van Cutsem E, et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420–35.
Lordick F, Kang Y, Chung H, Salman P, Oh S, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490–9.
Shah M, Bang Y, Lordick F, Alsina M, Chen M, Hack S, et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. 2017;3(5):620–7.
Kim C, Lee J, Ryu M, Chang H, Kim T, Lim H, et al. A prospective phase II study of cetuximab in combination with XELOX (capecitabine and oxaliplatin) in patients with metastatic and/or recurrent advanced gastric cancer. Invest New Drugs. 2011;29(2):366–73.
Wang Y, Cheng X, Cui Y, Hou J, Ji Y, Sun Y, et al. Efficacy after preoperative capecitabine and oxaliplatin (XELOX) versus docetaxel, oxaliplatin and S1 (DOS) in patients with locally advanced gastric adenocarcinoma: a propensity score matching analysis. BMC Cancer. 2018;18(1):702.
Zhong H, Zhang Y, Ma S, Ying J, Yang Y, Yong D, et al. Docetaxel plus oxaliplatin (DOCOX) as a second-line treatment after failure of fluoropyrimidine and platinum in Chinese patients with advanced gastric cancer. Anticancer Drugs. 2008;19(10):1013–8.
Luo H, Wang Z, Wang F, Qiu M, Teng K, Ruan D, et al. Phase 2 study of capecitabine and irinotecan combination chemotherapy (modified XELIRI regimen) in patients with advanced gastric cancer. Am J Clin Oncol. 2011;34(6):555–60.
Scott L. Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs. 2018;78(7):747–58.
Kim MA, et al. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52(6):738–46.
Tebbutt NC, et al. Docetaxel plus cetuximab as second-line treatment for docetaxel-refractory oesophagogastric cancer: the AGITG ATTAX2 trial. Br J Cancer. 2013;108(4):771–4.
Zhang ZD, et al. Clinical evaluation of cetuximab combined with an S-1 and oxaliplatin regimen for Chinese patients with advanced gastric cancer. World J Surg Oncol. 2014;12:115.
Arienti C, et al. Epidermal growth factor receptor family and its role in gastric cancer. Front Oncol. 2019;9:1308.
Rosfjord E, Lucas J, Li G, Gerber H. Advances in patient-derived tumor xenografts: from target identification to predicting clinical response rates in oncology. Biochem Pharmacol. 2014;91(2):135–43.
Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318–25.
Byrne A, Alférez D, Amant F, Annibali D, Arribas J, Biankin A, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17(4):254–68.
Reddavid R, Corso S, Moya-Rull D, Giordano S, Degiuli M. Patient-Derived Orthotopic Xenograft models in gastric cancer: a systematic review. Updat Surg. 2020;72(4):951–66.
Yang L, Yuan Z, Zhang Y, Cui Z, Li Y, Hou J, et al. MiniPDX-guided postoperative anticancer treatment can effectively prolong the survival of patients with hepatocellular carcinoma. Cancer Chemother Pharmacol. 2021;87(1):125–34.
Zhan M, Yang R, Wang H, He M, Chen W, Xu S, et al. Guided chemotherapy based on patient-derived mini-xenograft models improves survival of gallbladder carcinoma patients. Cancer Commun (London, England). 2018;38(1):48.
Zhao P, Chen H, Wen D, Mou S, Zhang F, Zheng S. Personalized treatment based on mini patient-derived xenografts and WES/RNA sequencing in a patient with metastatic duodenal adenocarcinoma. Cancer Commun. 2018;38(1):1–7.
Liu S, Liang H, Yang Z, Cai C, Wu Z, Wu X, et al. Gemcitabine and XCT790, an ERRα inverse agonist, display a synergistic anticancer effect in pancreatic cancer. Int J Med Sci. 2022;19(2):286–98.
Xu J, Gao Y, Luan X, Li K, Wang J, Dai Y, et al. An effective AKT inhibitor-PARP inhibitor combination therapy for recurrent ovarian cancer. Cancer Chemother Pharmacol. 2022. https://doi.org/10.1007/s00280-022-04403-9.
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. The Lancet. 2021;398(10294):27–40.
Acknowledgements
The authors would like to thank Dr. Danyi Wen form Shanghai LIDE Biotech Co., Ltd. and Geneplus-Beijing Institute for their assistance and technology supports.
Funding
This study was supported by J201801 from Precision Medicine Special project of Wuxi Municipal Health Commission.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: CJ, BZ; (II) Administrative support: CJ, BZ; (III) Collection and assembly of data: XZ, ZC, XH, TG and WZ; (IV) Data analysis and interpretation: YL, CZ, JQ, XL; (V) Manuscript writing: All authors; (VI) Final approval of manuscript: All authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study was performed in accordance with the Declaration of Helsinki regarding ethical principles for research involving human tissues. The protocols for our study were approved by the Institutional Review Board of Wuxi Hospital of Traditional Chinese Medicine (Ethical Approval Number 201809001J01-01). The animal study was reviewed and approved by the Institutional Animal care and use Committee(IACUC) Shanghai LIDI Biotech,Co.LTD, and written informed consent was obtained from all patients prior to participation in our study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, B., Li, Y., Zhu, X. et al. OncoVee™-MiniPDX-guided anticancer treatment for HER2-negative intermediate-advanced gastric cancer patients: a single-arm, open-label phase I clinical study. Discov Onc 14, 46 (2023). https://doi.org/10.1007/s12672-023-00661-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00661-y