Abstract
Background
Pancreatic cancer has a high mortality rate worldwide, and is predicted to be third leading cause of death in the near future. However, the regulatory mechanisms that inhibit the progression of pancreatic cancer remain elusive. Currently, exploring the function and mechanisms of GPCRs (G-protein coupled receptors) is an important way to discover promising therapeutic targets for cancer.
Methods
GPRC5A expression was measured using real-time quantitative PCR, immunohistochemistry and western blot assays. Cell proliferation and migration were assessed using CCK-8, clone formation, wound-healing and transwell assays. A cytosolic/nuclear distribution experiment was used to detect the protein location transfer. A xenograft model of pancreatic cancer was established to explore the role of GPRC5A in vivo.
Results
GPRC5A expression was increased in pancreatic cancer, and disruption of GPRC5A expression inhibited tumor growth in vivo. Mechanistically, GPRC5A positively regulated the transcription of YAP1 through cAMP-CREB signaling. Moreover, we show that the proliferation and migration induced by GPRC5A in pancreatic cancer could be rescued by inhibiting YAP1 expression.
Conclusions
GPRC5A interacts with the Hippo pathway to promote the progression of pancreatic cancer. These findings reveal an important crosstalk model and provide potential targets for pancreatic cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
The incidence of pancreatic cancer is increasing around the world [1]. Due to its high mortality rate, pancreatic cancer is currently the seventh leading cause of cancer deaths worldwide and may move up to the third in the near future [2]. Pancreatic ductal adenocarcinoma (PDAC) presently makes up the main diagnosed cases. As it is a fatal malignancy [3], it is urgent for more basic research to find new targets and bring new hope for pancreatic cancer treatment.
G-protein coupled receptors (GPCRs) are one of the largest membrane families that mediate extracellular signal transduction into cells and play a key role in many cell functions. GPCRs are one of the most promising therapeutic targets for cancer and targeting GPCR-disturbed cell signal transduction has become an important strategy for cancer drug development [4, 5]. Recent studies have shown that GPRC5A (G-Protein coupled Receptor Class C, Group 5, Member A) functions in the progression of a variety of cancer, including pancreatic cancer [6]. The expression and function of GPRC5A vary in cancers. For example, GPRC5A expression is reduced in lung cancer and inhibits lung tumorigenesis [7, 8], while in most cancers, GPRC5A functions as an oncogene. In pancreatic cancer, GPRC5A can indirectly interact with the RNA-binding protein HuR, which influences its translation [9]. GPRC5A may change the phosphorylation levels of STAT3 [10] and GSK-3β [11], through there is no evidence demonstrating that inhibiting the phosphorylation of these proteins blocks GPRC5A function. Therefore, it is still important to determine the key downstream molecules of GPRC5A and its mechanism of action in pancreatic cancer.
It is widely recognized that Hippo signaling is involved in the tumor suppressor pathway [12]. The transcriptional coactivator YAP1 is the core protein of the Hippo pathway, and its nuclear/cytoplasmic distribution is regulated by a serine/threonine kinase cascade that is launched through Hippo signaling activity [13]. Dysregulation of the Hippo pathway in pancreatic cancer is well known [14]. Multiple genes regulated by YAP1 are related to the unfavorable survival of pancreatic cancer patients, such as FOSL1 [15], CCND1 [16], and NOTCH2 [17]. GPCRs are an important type of upstream regulator that activate or inhibit the Hippo pathway [18]. In hypoxic situations, it has been reported that HIFs can reduce the phosphorylation level of YAP1 by directly promoting GPRC5A transcription [19]. However, the interaction between GPRC5A and YAP1 in pancreatic cancer is unclear.
In this study, we first address the roles of GPRC5A in nude mice and show that its overexpression in pancreatic cancer is predictive of poor prognosis. We demonstrate that GPRC5A regulates YAP1 expression at the transcription level but not at the activity level, which is governed by the phosphorylation kinase MST1/2 and LATS1/2. Importantly, YAP1 knockdown reversed the adverse effects of GPRC5A on pancreatic cancer cells. Moreover, we observed GPRC5A-influenced expression by cAMP-CREB signaling. We propose that the GPRC5A-cAMP-CREB axis is a crucial pathway in the promotion of pancreatic cancer progression.
2 Materials and methods
2.1 Cell lines and cell culture
The pancreatic cancer cell lines AsPC-1, BxPC-3, PANC1 and MIA PaCa-2 and human pancreatic ductal epithelial cell line hTERT-HPNE were obtained from the Beijing Beina Chuanglian Institute of Biotechnology (Beijing, China), cultured in DMEM (HyClone, Logan, UT, USA) containing 10% fetal bovine serum (FBS, HyClone, USA) and incubated at 37 °C with 5% CO2.
2.2 Plasmid, lentiviral infection, and transfection
The pcDNA3.1-GPRC5A plasmid was kindly provided by Prof. Jiong Deng (Shanghai Jiao Tong University, Shanghai, China). The GPRC5A plasmid (CMV-GPRC5A-EF1A-EGFP), GPRC5A knockdown lentivirus vector hU6-sh-GPRC5A-Ubi-EGFP (LV-sh-GPRC5A), and hU6-sh-Con-Ubi-EGFP empty lentiviral vector (LV-sh-NC) were synthesized by Genechem (Shanghai, China) [20]. The YAP1 plasmid (CMV-YAP1-T2A-EGFP), YAP1-targeting siRNA oligonucleotides were designed and synthesized by GenePharma (Shanghai, China). Cells were seeded on the plate one day before transfection and transfected using TurboFect transfection reagent (R0532; Thermo Scientific Scientific, Waltham, MA, United States). PANC1, MIA PaCa-2 and BxPC-3 cells were infected with lentivirus according to the manufacturer’s instructions. The sequences of the shRNA and siRNA used in our study were as follows: LV-sh-GPRC5A: 5’-CCTGACCATGAATAGGACCAA-3’, LV-sh-Con: TTCTCCGAACGTGTCACGT; si-YAP1: 5’-GGUGAUACUAUCAACCAAATT-3’, si-Con: UUCUCCGAACGUGUCAUGUTT.
2.3 Western blotting analysis
Total protein was extracted using RIPA buffer containing a protease inhibitor cocktail and phosphatase inhibitor cocktail (CWBIO, Jiangsu, China), separated by 10% SDS-PAGE, and then transferred to polyvinylidene difluoride membranes. The protein marker used in Western blotting was obtained from ThermoFisher Scientific (26616; ThermoFisher Scientific, Waltham, MA, United States). The membranes were blocked in 5% skim milk for 1 h at room temperature and then incubated at 4 °C with primary antibodies against GPRC5A (1:500; SC-373824; Santa Cruz), MST1 (1:500; ab51134; Abcam), MST2 (1:1000; #3952; Cell Signaling Technology), p-MST1/2 (1:500; 28953-1-AP; Proteintech), LATS1 (1:500; SC-398560; Santa Cruz), LATS2 (1:1,000; ab110780; Abcam), p-LATS1/2 (1:1,000; AF8163; Affinity), YAP1 (1:1,000; ab52771; Abcam), CYR61 (1:2,000; #14479; Cell Signaling Technology), Cyclin D1 (1:2,000; #2978; Cell Signaling Technology), c-Myc (1:2,000; #5605; Cell Signaling Technology), CTGF (1:2,000; #86641; Cell Signaling Technology), CREB (1:1,000; 13584-1-AP Proteintech), p-CREB (1:1,000; AP0019; ABclonal), GAPDH (1:10,000; 60004-1-Ig; Proteintech), or Histone-H3 (1:2,000; 17168-1-AP; Proteintech) overnight. The membranes were then washed three times with Tris-buffered saline with 0.1% Tween-20 (TBST buffer) and incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated secondary antibodies (ZSGB, China). After three final TBST washes, the resulting signals were detected via a chemiluminescence solvent (Thermo Fisher Scientific, Waltham, MA, USA).
2.4 Immunohistochemistry
Paraffin-embedded human pancreatic cancer tissues and adjacent normal tissues were collected from patients treated at the First Affiliated Hospital of Nanchang University. This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University. All paraffin-embedded tissues were deparaffinized with xylene, rehydrated with graded concentrations of alcohol, and subjected to antigen retrieval with citrate buffer. Endogenous peroxidase activity was blocked with 3% H2O2. Paraffin sections were incubated overnight with primary anti-GPRC5A or anti-YAP1 antibodies. Next, the sections were incubated with the appropriate secondary antibody, and DAB solution was added. All staining scores were assessed by two pathologists in a blinded manner based on staining intensity and positive staining ratio, and the grading standards were carried out as previously described [21].
2.5 RNA isolation and real-time quantitative PCR
Total RNA was extracted from cells or xenograft tumor tissues using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. cDNA was obtained by reverse transcription using PrimeScript™ RT reagent Kit with gDNA Erase (RR047A, Takara, Dalian, China). Real-time quantitative PCR (RT-PCR) was performed on an ABI Step One Plus system (Applied Biosystems, Foster City, CA, USA) using TB Green™ Premix Ex Taq II (RR820A, Takara, Dalian, China). Relative gene expression levels were normalized to GAPDH and calculated using the 2−ΔΔCT method. The primer sequences used are listed in Table 1.
2.6 RNA sequencing and analysis and bioinformatics analysis
Total RNA was extracted from PANC1 cells in the LV-sh-NC group or LV-sh-GPRC5A group using TRIzol Reagent. Partly isolated RNA samples were submitted to Beijing Genomics Institute for transcriptome sequencing following standard protocols and analysis on BGISEQ-500 [22]. Standard bioinformatics analysis was performed by the Beijing Genomics Institute. For gene expression analysis, the significance of the differentially expressed genes (DEGs) was defined by the bioinformatics service of BGI according to the combination of the absolute value of |log2(FC)|> 1 and adjusted p value < 0.05. GEPIA (http://gepia.cancer-pku.cn/) was used to determine and assess the expression and prognostic values of GPRC5A in pancreatic cancer. UALCAN (http://ualcan.path.uab.edu) was used to analyze the associations of GPRC5A with cancer stage and nodal metastasis status in pancreatic cancer. A p value < 0.05 was considered to be statistically significant.
2.7 Cell counting kit-8 (CCK-8) assay
Cells were plated in 96-well plates with 1 × 103 cells per well. Next, 100 μl FBS-free medium containing 10% CCK8 reagent was added to each well and incubated for 2 h at 37 °C. Finally, the absorbance of the cells was measured using a Varioskan LUX™ multimode microplate reader (Thermo Scientific) at 450 nm for 5 days. These experiments were conducted more than three times.
2.8 Colony formation assay
Cells were plated in 6-well plates with 2 × 103 cells per well. After 14 days, the colonies were fixed with methanol and stained with 1% crystal violet. The number of colonies was evaluated using ImageJ software.
2.9 Wound-healing assay
Cells were plated into 6-well plates with 5 × 105 cells per well until completely confluent. Straight wounds were scratched using a sterile 10 μl pipette tip and washed with phosphate-buffered saline three times. Next, the cells were incubated in serum-free medium for 24 h. Images were taken at 0 h and 24 h to calculate the wound closure percentage. Wound closure ranges in three randomly selected microscopic fields were counted for each group.
2.10 Transwell assays
Cells were plated into the upper compartment of the transwell chamber with 4 × 104 cells per well in 200 μl serum-free medium. The bottom chamber contained 600 μl DMEM with 20% FBS. After 48 h, cells that migrated through the chamber membranes were fixed with methanol and stained with 1% crystal violet.
2.11 Animal models
All animal experiments were performed using protocols approved by the Animal Center of The First Affiliated Hospital of Nanchang University. Six-week-old female nude mice were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Hunan, China), and 1 × 107 LV-sh-NC or LV-sh-GPRC5A BxPC-3 cells were subcutaneously inoculated into the left axilla of each mouse. Tumor growth was measured every week with electronic digital calipers (Thermo Scientific). Tumor volume was calculated with the formula: tumor volume (mm3) = [length × (width)2]/2. After 5 weeks, the xenograft tumors were harvested and weighed.
2.12 Statistical analysis
All the data are presented as the mean ± standard deviation. The Student’s t-test, one-way ANOVA, two-way ANOVA, and log-rank (Mantel-Cox) test were adopted for the statistical analyses. P < 0.05 was considered statistically significant. * indicates P < 0.05, ** indicates P < 0.01, and *** indicates P < 0.001.
3 Results
3.1 Upregulation of GPRC5A predicts poor prognosis in pancreatic cancer
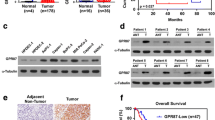
To further demonstrate the role of GPRC5A in pancreatic cancer, we detected its expression in pancreatic cancer cells and tissues. We first examined GPRC5A expression in pancreatic cancer cell lines and normal cells at the protein and mRNA levels, and observed that GPRC5A expression was higher in pancreatic cancer cells than in normal cells (Fig. 1A, B). GPRC5A was significantly upregulated in pancreatic cancer compared to normal tissues analyzed through the TCGA and GEPIA databases (Fig. 1C, D). Based on cancer stages, compared with the normal group, we found that patients in higher stages had more GPRC5A expression. In addition, an increasing trend of GPRC5A expression was observed from stage I to stage IV (Fig. 1E). In terms of nodal metastasis status, both N0 and N1 had significantly greater expression of GPRC5A than the normal group (Fig. 1F). In addition, we observed increased GPRC5A expression in the tumor tissues in contrast to normal tissues in pancreatic cancer patients by IHC (Fig. 1G). Similarly, the GPRC5A protein level was higher in pancreatic cancer tissues than in adjacent normal tissues (Fig. 1H). Last, we found that upregulated GPRC5A predicted poor overall survival (OS) and disease-free survival (DFS) using the GEPIA database (Fig. 1I, J). Thus, these results suggested that GPRC5A is overexpressed in pancreatic cancer tissues and cells and predicts a poor prognosis for pancreatic cancer patients.
GPRC5A is upregulated in pancreatic cancer and predicts poor prognosis in pancreatic cancer patients. A, B The protein (A) and mRNA (B) expression level of GPRC5A in pancreatic cancer cell lines and normal hTERT-HPNE cells by Western blotting and RT-PCR. C, D Analysis of GPRC5A mRNA relative expression in the TCGA (C) and GEPIA (D) databases. Student’s t test, *p < 0.05, ***p < 0.001. E, F Association of GPRC5A expression with cancer stage (E) and nodal metastasis status (F) in pancreatic cancer patients. One-way ANOVA, post hoc, Bonferroni, ***p < 0.001. G, H Representative images of IHC staining of GPRC5A in human pancreatic cancer tissues and normal tissue (G) and adjacent normal tissue samples (H). Red lines represent 200 μm, and blank lines represent 50 μm. I, J The overall survival (I) and disease-free survival (J) of pancreatic cancer patients treated with GPRC5A were obtained using the GEPIA database. Log-rank (Mantel-Cox) test
3.2 Knockdown of GPRC5A inhibits cell proliferation and migration of pancreatic cancer in vitro
To explore the efficiency of GPRC5A shRNA, the whole transcriptome profiles of PANC1 cells with LV-sh-NC or LV-sh-GPRC5A were analyzed by RNA-seq and confirmed in PANC1 and MIA PaCa-2 cells by Western blotting (Fig. 2A, B). Then, we performed functional experiments to assess the role of GPRC5A in pancreatic cancer cells. Colony formation assays revealed that GPRC5A knockdown markedly decreased cell growth in PANC1 and MIA PaCa-2 cells (Fig. 2C, D). Interestingly, GPRC5A knockdown also markedly decreased the migration ability of pancreatic cancer cells (Fig. 2E, F). Taken together, our data indicate that GPRC5A knockdown suppresses pancreatic cancer cell proliferation and migration in vitro.
Knockdown of GPRC5A inhibits cell proliferation and migration of pancreatic cancer in vitro. A, B PANC1 and MIA PaCa-2 cells were transfected with GPRC5A shRNA, and the efficiency was detected by Western blotting. C, D Colony formation assay was used to evaluate cell proliferation. Student’s t test, ***p < 0.001. E, F Transwell assay was used to evaluate migration ability. Scale bar = 50 μm. Student’s t test, ***p < 0.001
3.3 GPRC5A promotes the growth of pancreatic cancer in vivo
To investigate the effect of GPRC5A on tumor growth, we next established a subcutaneous xenograft tumor model. Pancreatic cancer cells Infected with LV-sh-NC or LV-sh-GPRC5A were inoculated into nude mice, and tumor size was monitored for 5 weeks. The knockdown efficiency of GPRC5A was analyzed using RT-PCR and Western blotting (Fig. 3A, B). The volume of the tumors was significantly reduced in the LV-sh-GPRC5A group compared to the LV-sh-NC group from the first week to the fifth week (Fig. 3C). After 5 weeks of implantation, the tumor parts were directly inspected or dissected from the nude mice to measure the volume and weight (Fig. 3D–F). The tumor weight was significantly decreased in the LV-sh-GPRC5A group as opposed to the LV-sh-NC group in the fifth week (Fig. 3G). To confirm the expression of GPRC5A, xenograft tumors were identified by performing RT-PCR and Western blotting. As expected, the mRNA and protein levels of GPRC5A were significantly decreased in the LV-sh-GPRC5A group (Fig. 3H, I). Collectively, our data demonstrate that GPRC5A knockdown inhibits the growth of implanted tumors in vivo.
GPRC5A promotes the growth of pancreatic cancer in vivo. A, B The knockdown efficiency was detected by RT-PCR and Western blotting in BxPC-3 cells infected with LV-sh-NC or LV-sh-GPRC5A. Student’s t test, ***p < 0.001. C Weekly volume change in nude mice after tumors implantation. Two-way ANOVA, post hoc, Bonferroni, ***p < 0.001. D–F Tumor volume images are shown in the LV-sh-GPRC5A group and LV-sh-NC group in nude mice at the fifth week. n = 6. G Quantification of the average weights of tumor tissues dissected from the nude mice. Student’s t test, **p < 0.01. H, I The mRNA and protein levels of GPRC5A were detected by RT-PCR and Western blotting. Student’s t test, *p < 0.05
3.4 GPRC5A dysregulates the Hippo pathway by regulating the YAP1 transcript
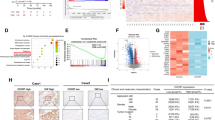
To explore the potential molecular mechanism that was responsible for the effects of GPRC5A on pancreatic cancer progression, the whole transcriptome profiles of PANC1 cells with LV-sh-NC group or LV-sh-GPRC5A group were analyzed by RNA-seq. We screened out DEGs in LV-sh-GPRC5A group compared to the LV-sh-NC group with the criteria of |log2(FC)|> 1 and adjusted p value < 0.05, which included 413 upregulated genes and 247 downregulated genes (Fig. 4A). Among them, we further selected 192 DEGs with the criteria of |log2(FC)|> 2 displayed on the heat map (Fig. 4B). KEGG enrichment analyses showed that the DEGs were enriched in cancer-related pathways, including Hippo signaling pathway, p53 signaling pathway, PI3K-Akt signaling pathway TGF-beta signaling pathway (Fig. 4C). Disruption of Hippo signaling is common in pancreatic cancer [14], and has an important influence on pancreatic cancer tumorigenesis and progression [23, 24]. Previous research has shown that GPRC5A promotes cancer cell adaptation to hypoxia by activating YAP1, the core protein of the Hippo pathway [19]. To investigate whether GPRC5A promoted pancreatic cancer progression via the YAP1 pathway, we evaluated the relationship between GPRC5A and YAP1 in pancreatic cancer tissues and cells. We first found a significantly positive relationship between GPRC5A and YAP1 in pancreatic cancer using the GEPIA database (Fig. 4D). Next, we examined YAP1 expression in pancreatic cancer cell lines and normal cells at both the mRNA and protein levels and found that the expression of YAP1 was positively related to GPRC5A expression (Fig. 4E, F). Correspondingly, GPRC5A and YAP1 had similar expression patterns in tumor tissues (Fig. 4G, H).
GPRC5A is positively correlated with YAP1 in pancreatic cancer. A The volcano plot presents the GPRC5A-related differentially expressed genes (DEGs) by RNA sequencing analysis. Red and green dots represent up and downregulated genes, respectively. B Heat map and hierarchical clustering based on the most differentially expressed genes screened out with the criteria of |log2(FC)|> 2 and adjusted p value < 0.05. C KEGG pathway enrichment analysis was used to identify the top 20 cancer-related pathways with significant enrichment. D The correlation between GPRC5A and YAP1 in pancreatic cancer was evaluated using the GEPIA database. E The protein and mRNA expression levels of YAP1 in pancreatic cancer cell lines and normal hTERT-HPNE cells by Western blotting and RT-PCR. F The correlation between GPRC5A and YAP1 was detected in the mRNA and protein levels in pancreatic cancer cell lines and normal hTERT-HPNE cells. G Representative images of GPRC5A and YAP1 in human pancreatic cancer tissues and adjacent normal tissue samples as shown by IHC staining. Scale bar = 50 μm. H The IHC scores of GPRC5A and YAP1 in human pancreatic cancer tissues. Student’s t test, *p < 0.05, ***p < 0.001
To explore the impact of GPRC5A on YAP1, we evaluated the relationship between GPRC5A and YAP1 downstream target genes in pancreatic cancer patients. The results showed that GPRC5A was closely correlated with CYR61, cyclin D1, c-Myc, and CTGF in pancreatic cancer (Fig. 5A–D). We then examined the protein expression of the Hippo pathway after bidirectional control of GPRC5A. The results showed that knockdown or overexpression of GPRC5A prominently decreased or increased, respectively, the expression of YAP1 and its target genes, CYR61, cyclin D1, c-Myc and CTGF (Fig. 5E, F, I, J). Regulation of GPRC5A expression did not impact on MST1/2, LATS1/2 and its phosphorylated states, which is the upstream kinase that regulates the YAP1 activity [25] (Fig. 5E, F, I, J). In addition, YAP1 and p-YAP1 changed synchronously without a significant change in the ratio between YAP1 and p-YAP1 based on the regulation of GPRC5A expression (Fig. 5G, K). Moreover, we examined the mRNA expression of YAP1 and its downstream genes based on modulation of GPRC5A expression. The results showed that GPRC5A positively regulated YAP1, CYR61, cyclin D1, c-Myc and CTGF expression at the transcriptional level, as evidenced by overexpression and knockdown of GPRC5A in pancreatic cancer cells (Fig. 5H, L). Taken together, our results show that GPRC5A dysregulates Hippo signaling by regulating YAP1 transcription.
GPRC5A modulates the Hippo pathway by mediating YAP1 transcription. A–D The correlation between GPRC5A and CYR61, cyclin D1, c-Myc and CTGF in pancreatic cancer was evaluated using the GEPIA database. E, F Western blotting analysis of MST1/2, p-MST1/2, LATS1/2, p-LATS1/2, YAP1, p-YAP1, CYR61, cyclin D1, c-Myc and CTGF expression after GPRC5A downregulation. Student’s t test, *p < 0.05, **p < 0.01, ***p < 0.001. G The ratio between YAP1 and p-YAP1 after downregulation of GPRC5A. H RT-PCR analysis of YAP1, CYR61, cyclin D1, c-Myc and CTGF after downregulation of GPRC5A. Student’s t test, *p < 0.05, **p < 0.01, ***p < 0.001. I-J Western blotting analysis of MST1/2, p-MST1/2, LATS1/2, p-LATS1/2, YAP1, p-YAP1, CYR61, cyclin D1, c-Myc and CTGF expression after GPRC5A upregulation. Student’s t test, *p < 0.05, **p < 0.01, ***p < 0.001. K The ratio between YAP1 and p-YAP1 after upregulation of GPRC5A. L RT-PCR analysis of YAP1, CYR61, cyclin D1, c-Myc and CTGF after upregulation of GPRC5A. Student’s t test, *p < 0.05, **p < 0.01, ***p < 0.001
3.5 YAP1 interference inhibits the effects of GPRC5A on the proliferation and migration of pancreatic cancer cells
To further determine the role of YAP1 in GPRC5A-mediated function in pancreatic cancer, we upregulated YAP1 using YAP1 plasmid with or without downregulation of GPRC5A in pancreatic cancer cells. Upregulating YAP1 only increased its expression without influencing GPRC5A expression in PANC1 cells (Supplementary Figure S1A, B). In addition, YAP1 overexpression partially counteracted the effects of GPRC5A knockdown (Supplementary Figure S1A, B). Interestingly, we downregulated YAP1 using siRNA with or without GPRC5A overexpression in pancreatic cancer cells. Disrupting YAP1 only reduced its expression without influencing GPRC5A expression in PANC1 cells (Fig. 6A, B). In addition, YAP1 deficiency partially counteracted the effects of GPRC5A upregulation of YAP1 and its target gene CYR61 (Fig. 6A, B). Furthermore, the influence of GPRC5A on promoting the proliferation and migration of pancreatic cancer cells was partially reversed by YAP1 deficiency in PANC1 cells, which was verified by CCK-8, colony formation, transwell, and wound healing assays (Fig. 6C–I). In summary, our findings demonstrated that inhibiting YAP1 was beneficial in disrupting the adverse effects of GPRC5A on pancreatic cancer cells.
YAP1 interference inhibits the effects of GPRC5A on the proliferation and migration of pancreatic cancer cells. A, B PANC1 cells were transfected with GPRC5A or control plasmids combined with YAP1 siRNA or si-Con, and Western blotting was performed to detect the expression of GPRC5A, YAP1 and its target gene CYR61. One-way ANOVA, post hoc, Bonferroni, *p < 0.05, **p < 0.01, ***p < 0.001, (vs. Control + si-Con); #p < 0.05, ##p < 0.01, ###p < 0.001, (vs. GPRC5A + si-Con); &p < 0.05, &&&p < 0.001, (vs. Control + si-YAP1). C–E Cell proliferation was evaluated using CCK-8 (C) and colony formation assays (D, E) performed on GPRC5A-overexpressing cells with YAP1 knockdown. Two-way ANOVA and one-way ANOVA, post hoc, Bonferroni, ***p < 0.001, (vs. Control + si-Con); ##p < 0.01, ###p < 0.001, (vs. GPRC5A + si-Con); &&p < 0.01, &&&p < 0.001, (vs. Control + si-YAP1). F–I Cell migration ability was evaluated via transwell (F, G) and wound-healing assays (H, I) after GPRC5A overexpression with YAP1 knockdown. Scale bar = 50 μm. One-way ANOVA, post hoc, Bonferroni, **p < 0.01, ***p < 0.001, (vs. Control + si-Con); ##p < 0.01, ###p < 0.001, (vs. GPRC5A + si-Con); &&p < 0.01, &&&p < 0.001, (vs. Control + si-YAP1)
3.6 GPRC5A mediates the Hippo-YAP1 pathway through cAMP-CREB signaling
It has been previously reported that GPCRs promote the generation of cAMP (cyclic adenosine monophosphate), which is vital for the progression of physiology and pathology [26, 27]. Thus, we investigated cAMP expression after regulating GPRC5A in pancreatic cancer cells. The results showed GPRC5A knockdown decreased the mRNA expression of cAMP (Fig. 7A). Moreover, GPRC5A overexpression increased cAMP expression (Fig. 7B). cAMP-PKA-CREB signaling plays an important role in cancer cell growth, migration, and metabolism [28]. CREB is activated by Ser133 phosphorylation and then binds to YAP1 promoter areas to upregulate its transcription [29, 30] Therefore, we investigated whether GPRC5A controlled YAP1 expression by cAMP/CREB pathways. To test this hypothesis, we first measured the effect of GPRC5A on CREB activity, and our results indicated that silencing or overexpressing GPRC5A drastically increased or decreased the phosphorylation of CREB without significantly affecting total CREB expression (Fig. 7C, D). Intriguingly, we observed that nuclear localization of phosphorylated CREB was upregulated and cytoplasmic phosphorylated CREB expression was downregulated upon GPRC5A overexpression (Fig. 7F). Conversely, GPRC5A knockdown decreased nuclear phosphorylated CREB levels and promoted cytoplasmic phosphorylated CREB expression (Fig. 7E). Taken together, these data suggested that GPRC5A disrupted the Hippo-YAP1 pathway through cAMP-CREB signaling (Fig. 7G).
GPRC5A mediates the Hippo-YAP1 pathway through the cAMP-CREB axis. A, B RT-PCR analysis of cAMP expression after GPRC5A was downregulated or upregulated. Student’s t test, **p < 0.01, ***p < 0.001. C, D Western blotting was performed to detect p-CREB and CREB expression following GPRC5A knockdown or overexpression. E, F Western blotting showing the distribution of p-CREB in the cytoplasmic and nuclear extracts follwing modification of GPRC5A expression in pancreatic cancer cells. G The proposed working model of GPRC5A promoting pancreatic cancer cell proliferation and migration by disrupting the Hippo pathway through cAMP-CREB signaling
4 Discussion
Aberrant expression of GPRC5A is usually positive or negative in controlling the process of tumorigenesis [6], an effect that depends on its expression level and downstream regulatory molecules. Approximately half of the studies on GPRC5A have focused on the lung, and GPRC5A acts as a tumor suppressor in lung cancer with low expression [31, 32]. In most reported cancers, GPRC5A promotes cancer progression regardless of its up- or downregulation in cells [6]. In our experiments, GPRC5A was markably increased in pancreatic cancer compared to normal or adjacent tissue, and we first confirmed that in pancreatic cancer, GPRC5A interference inhibited tumor growth in nude mice.
Although two papers have explored GPRC5A function in pancreatic cancer [10, 11], they did not provide evidence from in vivo experiments or show that blocking downstream molecules disrupted the effect of GPRC5A. Our experiments showed that GPRC5A could bidirectionally control the expression of YAP1 and its target genes at the transcriptional level in pancreatic cancer cells. However, this is very different from studies in hypoxic conditions, which showed that GPRC5A influences the phosphorylation of LATS1/2 kinases to regulate YAP1 activity [19]. YAP1 activity, particularly its phosphorylation level, is commonly regulated by the LATS1/2 kinase of the Hippo pathway and is critical for tumor initiation, progression, and metastasis [33, 34]. Our results suggest that MST1/2 and LATS1/2 protein did not change markedly based on modulation of GPRC5A expression, which indicated that the regulation of YAP1 by GPRC5A is independent of LATS1/2 kinase in pancreatic cancer cells. Furthermore, our data showed that silencing YAP1 by siRNA disrupted the malignant biological behavior of GPRC5A in pancreatic cancer cells. These findings reveal an important crosstalk model between GPRC5A and the Hippo pathway in pancreatic cancer progression.
Activation of GPCRs always results in increased cAMP [35]. CREB activity is a marker of cAMP accumulation and a prominent direct target of PKA. It is well known that cAMP-PKA-CREB signaling participates in tumorigenesis [28]. Here, we demonstrated that GPRC5A upregulated cAMP and promoted CREB phosphorylation. One previous study showed that the cAMP-PKA axis also phosphorylates YAP1, through the response time lasted only 1 h ~ 4 h [36]. Our experiments demonstrated that disrupting YAP1 expression almost completely inhibited the effect of GPRC5A on pancreatic cancer cells. GPCRs play important roles in pathophysiology and pharmacological tractability; thus, GPCRs have been deemed to be one of the most valuable therapeutic targets for cancer treatment [37]. Moreover, the success rates of GPCR agents in phases I, II, and III were moderately higher than FDAs-approved average for all examined agents. However, compounds that disrupt GPCR-mediated pathways are limited in clinical application [4]. It is meaningful to explore the structure of GPRC5A and determine agonists or inhibitors to investigate its effectiveness in clinical trials for cancer treatment.
5 Conclusions
Generally, the GPRC5A working model varies in different cancers. In this study, we demonstrated that crosstalk between the Hippo pathway and GPRC5A-cAMP-CREB signaling was critical for pancreatic cancer progression. This finding suggests that the GPRC5A-cAMP-CREB-YAP1 axis may provide candidate targets for therapeutic application.
Data availability
All the datasets generated and analyzed in the present study are available from the corresponding author on reasonable request.
Abbreviations
- GPCRs:
-
G-protein coupled receptors
- GPRC5A:
-
G-Protein coupled Receptor Class C, Group 5, Member A
- PDAC:
-
Pancreatic ductal adenocarcinoma
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- cAMP:
-
Cyclic adenosine monophosphate
- DEGs:
-
Differentially expressed genes
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Liu Y, An S, Ward R, Yang Y, Guo XX, Li W, Xu TR. G protein-coupled receptors as promising cancer targets. Cancer Lett. 2016;376(2):226–39.
Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10(1):47–60.
Jiang X, Xu X, Wu M, Guan Z, Su X, Chen S, Wang H, Teng L. GPRC5A: an emerging biomarker in human cancer. Biomed Res Int. 2018;2018:1823726.
Zhong S, Yin H, Liao Y, Yao F, Li Q, Zhang J, Jiao H, Zhao Y, Xu D, Liu S, et al. Lung tumor suppressor GPRC5A binds EGFR and restrains its effector signaling. Cancer Res. 2015;75(9):1801–14.
Wang T, Jing B, Xu D, Liao Y, Song H, Sun B, Guo W, Xu J, Li K, Hu M, et al. PTGES/PGE2 signaling links immunosuppression and lung metastasis in Gprc5a-knockout mouse model. Oncogene. 2020;39(15):3179–94.
Zhou H, Telonis AG, Jing Y, Xia NL, Biederman L, Jimbo M, Blanco F, Londin E, Brody JR, Rigoutsos I. GPRC5A is a potential oncogene in pancreatic ductal adenocarcinoma cells that is upregulated by gemcitabine with help from HuR. Cell Death Dis. 2016;7: e2294.
Jahny E, Yang H, Liu B, Jahnke B, Lademann F, Knosel T, Rummele P, Grutzmann R, Aust DE, Pilarsky C, et al. The G protein-coupled receptor RAI3 is an independent prognostic factor for pancreatic cancer survival and regulates proliferation via STAT3 phosphorylation. PLoS ONE. 2017;12(1): e0170390.
Liu B, Yang H, Pilarsky C, Weber GF. The effect of GPRC5a on the proliferation, migration ability, chemotherapy resistance, and phosphorylation of GSK-3beta in pancreatic cancer. Int J Mol Sci. 2018;19(7):1870.
Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246–57.
Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30(1):1–17.
Rozengurt E, Sinnett-Smith J, Eibl G. Yes-associated protein (YAP) in pancreatic cancer: at the epicenter of a targetable signaling network associated with patient survival. Signal Transduct Target Ther. 2018;3:11.
Vallejo A, Perurena N, Guruceaga E, Mazur PK, Martinez-Canarias S, Zandueta C, Valencia K, Arricibita A, Gwinn D, Sayles LC, et al. An integrative approach unveils FOSL1 as an oncogene vulnerability in KRAS-driven lung and pancreatic cancer. Nat Commun. 2017;8:14294.
Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17(9):1218–27.
Wu N, Nguyen Q, Wan Y, Zhou T, Venter J, Frampton GA, DeMorrow S, Pan D, Meng F, Glaser S, et al. The Hippo signaling functions through the Notch signaling to regulate intrahepatic bile duct development in mammals. Lab Invest. 2017;97(7):843–53.
Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780–91.
Greenhough A, Bagley C, Heesom KJ, Gurevich DB, Gay D, Bond M, Collard TJ, Paraskeva C, Martin P, Sansom OJ, et al. Cancer cell adaptation to hypoxia involves a HIF-GPRC5A-YAP axis. EMBO Mol Med. 2018. https://doi.org/10.15252/emmm.201708699.
Wang K, Huang W, Chen R, Lin P, Zhang T, Ni YF, Li H, Wu J, Sun XX, Geng JJ, et al. Di-methylation of CD147-K234 promotes the progression of NSCLC by enhancing lactate export. Cell Metab. 2021;33(1):160-173 e166.
Yao Y, Liu Z, Huang S, Huang C, Cao Y, Li L, Guo H, Liu F, Huang S, Liao Q, et al. The E3 ubiquitin ligase, FBXW5, promotes the migration and invasion of gastric cancer through the dysregulation of the Hippo pathway. Cell Death Discov. 2022;8(1):79.
Wang J, Chen M, Wang M, Zhao W, Zhang C, Liu X, Cai M, Qiu Y, Zhang T, Zhou H, et al. The novel ER stress inducer Sec C triggers apoptosis by sulfating ER cysteine residues and degrading YAP via ER stress in pancreatic cancer cells. Acta Pharm Sin B. 2022;12(1):210–27.
Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7(324):ra42.
Zhang Q, Zhang Y, Parsels JD, Lohse I, Lawrence TS, Pasca di Magliano M, Sun Y, Morgan MA. Fbxw7 deletion accelerates Kras(G12D)-driven pancreatic tumorigenesis via Yap accumulation. Neoplasia. 2016;18(11):666–73.
Zheng Y, Pan D. The Hippo signaling pathway in development and disease. Dev Cell. 2019;50(3):264–82.
Kimple ME, Keller MP, Rabaglia MR, Pasker RL, Neuman JC, Truchan NA, Brar HK, Attie AD. Prostaglandin E2 receptor, EP3, is induced in diabetic islets and negatively regulates glucose- and hormone-stimulated insulin secretion. Diabetes. 2013;62(6):1904–12.
Ali DC, Naveed M, Gordon A, Majeed F, Saeed M, Ogbuke MI, Atif M, Zubair HM, Changxing L. beta-Adrenergic receptor, an essential target in cardiovascular diseases. Heart Fail Rev. 2020;25(2):343–54.
Zhang H, Kong Q, Wang J, Jiang Y, Hua H. Complex roles of cAMP-PKA-CREB signaling in cancer. Exp Hematol Oncol. 2020;9(1):32.
Zhang T, Zhang J, You X, Liu Q, Du Y, Gao Y, Shan C, Kong G, Wang Y, Yang X, et al. Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology. 2012;56(6):2051–9.
Wang J, Ma L, Weng W, Qiao Y, Zhang Y, He J, Wang H, Xiao W, Li L, Chu Q, et al. Mutual interaction between YAP and CREB promotes tumorigenesis in liver cancer. Hepatology. 2013;58(3):1011–20.
Deng J, Fujimoto J, Ye XF, Men TY, Van Pelt CS, Chen YL, Lin XF, Kadara H, Tao Q, Lotan D, et al. Knockout of the tumor suppressor gene Gprc5a in mice leads to NF-kappaB activation in airway epithelium and promotes lung inflammation and tumorigenesis. Cancer Prev Res (Phila). 2010;3(4):424–37.
Tao Q, Fujimoto J, Men T, Ye X, Deng J, Lacroix L, Clifford JL, Mao L, Van Pelt CS, Lee JJ, et al. Identification of the retinoic acid-inducible Gprc5a as a new lung tumor suppressor gene. J Natl Cancer Inst. 2007;99(22):1668–82.
Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283(9):5496–509.
Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J Biol Chem. 2008;283(41):27534–46.
Cho-Chung YS. Role of cyclic AMP receptor proteins in growth, differentiation, and suppression of malignancy: new approaches to therapy. Cancer Res. 1990;50(22):7093–100.
Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27(11):1223–32.
Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16(12):829–42.
Acknowledgements
We thank Prof. Jiong Deng (Shanghai Jiao Tong University, Shanghai, China) for providing the pcDNA3.1-GPRC5A plasmid.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Numbers 82001159 and 82160459), the Natural Science Foundation of Jiangxi Province (Grant Numbers 20224BAB206058, 20212BAB216035, 20202BCD42011 and 20192ACB20028), and the Science and Technology Program of Jiangxi Provincial Health Commission (Grant Number 202210369).
Author information
Authors and Affiliations
Contributions
MM and BY initiated and designed the study. WF and XY performed the experiments. MM, WF and BY wrote the manuscript. MM, WF and XY analyzed the data. JD and JX supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The First Affiliated Hospital of Nanchang University. Informed consent was obtained from each patient prior to their inclusion in the study.
Ethics approval of animals
All animal procedures were performed according to the protocol approved by The First Affiliated Hospital of Nanchang University. We declared that the maximal tumor size was less than 15 mm in diameter in all dimensions in mice. The study complies with the Ethics and Guidelines for Animal Research and was approved by The First Affiliated Hospital of Nanchang University. This study follows the legal requirements or guidelines for the care and use of animals in China, and the International Union for Conservation of Nature (IUCN) Policy Statement on Research Involving Species at Risk of Extinction.
Consent for publication
All authors consent to publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12672_2023_626_MOESM1_ESM.tif
Supplementary Figure S1. Western blotting showed the expression of GPRC5A, YAP1 and CYR61 after transfecting YAP1 or control plasmids combined with LV-sh-NC or LV-sh-GPRC5A in PANC1 cells. One-way ANOVA, post hoc, Bonferroni, *p < 0.05, **p < 0.01, ***p < 0.001, (vs. LV-sh-NC+OE-NC); ##p < 0.01, ###p < 0.001, (vs. LV-sh-GPRC5A+ OE-NC); &p < 0.05, &&p < 0.01, (vs. LV-sh-NC+OE-YAP1). (TIF 4529 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, W., Yu, X., Deng, J. et al. Upregulated GPRC5A disrupting the Hippo pathway promotes the proliferation and migration of pancreatic cancer cells via the cAMP-CREB axis. Discov Onc 14, 17 (2023). https://doi.org/10.1007/s12672-023-00626-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00626-1