Abstract
Background
Menstrual and parity history might impact the risk for breast cancer. Data on the impact of these factors on other tumor characteristics are limited.
Methods
A single center retrospective cohort study comprising all women with estrogen receptor (ER) positive, human epidermal growth factor receptor 2 (HER2) negative, early breast cancer whose tumors were sent to OncotypeDX analysis. The prespecified subgroups were investigated: age of menarche (< 12 vs. ≥ 12 years), number of deliveries (0 vs. ≥ 1 childbirth and ≥ 5 childbirth vs. other), age of first delivery (≥ 30 years vs. younger age) and postmenopausal compared to premenopausal. The impact of age of menopause was also assessed categorically, using early (< 45 years) and late age of menopause (> 55 years). Differences in tumor characteristics were evaluated using T-test or Mann Whitney for continuous variables or Fisher’s exact test for categorical variables. Outcomes were assessed by Kaplan–Meier survival analysis, with the log-rank test.
Results
A total of 620 women were included. After median follow-up of 10.4 years, early menopause was associated with significantly worse disease-free survival (HR = 2.26, p = 0.004) and overall-survival (HR = 2.60, p = 0.004), and multiparity was associated with significant worse disease-free survival (HR = 2.16, p = 0.026). These differences remain significant in multivariate analyses. Post-menopausal women were more likely to have stronger ER intensity (p = 0.002) but progesterone receptor (PR) positivity was less frequent (p = 0.009(. Early age of menarche was associated with PR positivity (p = 0.039). No other associations were found between the evaluated subgroups and tumor characteristics.
Conclusions
The impact of endogenous estrogen exposure had little effect on breast cancer characteristics of early stage, luminal disease. Early menopause and multiparity were associated with worse outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Breast cancer accounts for 30% of all cancer diagnosed in women, with a lifetime risk of 12.4% [1]. Estrogen and its metabolites have a role in breast cancer development. Exposure to high levels of either endogenous or exogenous estrogen increase the risk of breast cancer, particularly estrogen receptor (ER) positive breast cancer [2], which is overexpressed in approximately 75% of all breast cancers [3]. In contrast to exogenous estrogen exposure, which is determined by treatment women might take during their life time such as oral contraceptive or hormone replacement therapy, endogenous estrogen exposure is determined by events women are experiencing during their life time, including duration of ovulations (i.e. time between menarche and menopause) and pregnancies.
Excess risk of breast cancer has been attributed to early menarche and late menopause, this is associated to lengthening woman’s reproductive years [4]. Compared to early menarche, late menarche is associated with decreased risk for breast cancer which is more robust for ER positive disease than ER negative disease [5]. Late menopause independently increases the risk for breast cancer, with a relative increase of 3% for every year older at menopause [4].
Parity also effects breast cancer risk through hormonal mechanisms. Nulliparity is related to an increase risk of breast cancer, whereas multiparity has an overall protective effect, although there is an increased risk for breast cancer during the first two decades after childbirth and then it gradually decreases [6, 7]. Age at first full-term pregnancy is another factor related to breast cancer risk, with lower breast cancer risk in women with younger age of first birth [8]. Obesity is a known risk factor for cancer, higher BMI increases the risk for ER positive breast cancer specifically in postmenopausal women, possibly explained by higher estrogen levels resulting from the peripheral conversion of estrogen precursors from adipose tissue [9, 10].
The impact of endogenous estrogen on breast cancer characteristics is less known. Earlier age at menarche and older age of menopause are associated with more frequently lobular tumors [4]. Older age at menopause is associated with the development of ER positive tumors rather than to ER negative tumors [4]. An association between the baseline levels of sex-hormone has also been described in postmenopausal women, with higher level of estradiol and testosterone associated with increased risk of ER positive disease, but not with ER negative breast cancer [11, 12].
Breast cancer is a diversified disease, with different histopathological characteristics and molecular subtypes that determine both treatment and prognosis [13]. ER positive, human epidermal growth factor receptor 2 (HER2) negative-subtype represents approximately 65–70% of invasive breast cancer and compared to other subtypes have a better prognosis [13]. Yet, the luminal-subtype has a spectrum of variables affecting its prognosis and treatment, including the extent of the disease, grade, Ki67, intensity of ER and genomic risk [14,15,16,17]. One of the genomic assays used in this population is the 21-gene recurrence-score assay (Oncotype DX, genomic Health) which provides both predictive and prognostic information in early-stage luminal disease [18].
The aim of our study was to investigate the effect of endogenous estrogen on histological tumor characteristics and on the genomic risk in early breast cancer women with ER positive, HER2 negative disease and to explore the correlation of these exposures on outcomes.
2 Methods
This was a retrospective single center cohort study. All women who were treated in our institute between 2005 and 2012 with ER positive, HER2 negative, early breast cancer whose tumors were sent to Oncotype DX analysis were included. As Oncotype DX was available and reimbursed in Israel since 2005, nearly all patients with luminal early breast cancer who were medically fit for adjuvant chemotherapy were sent for Oncotype DX. The Clalit Heath Services are the medical provider for the majority of the included patients in this cohort.
A detailed review of patients’ medical records and pathological reports was conducted. Data on demographics, adjuvant therapy and pre-specified clinical-pathological parameters were extracted including: tumor size (categorized as T ≤ 1 cm, 1 < T ≤ 2 cm and T > 2 cm), nodal status (negative or positive, including both macro- and micrometastases), intensity of ER and progesterone receptor (PR) expression, grade, Ki67, lymphovascular and perineural invasion and Oncotype DX recurrence score (RS). RS was categorized by the TailorX groups: low risk: RS ≤ 25 and high risk: RS > 25 [20]. ER/PR staining report was based on the modified version of H-score method [21] [(1× % cells + 1) + (2× % cells + 2) + (3× % cells + 3)]/100, yielding a score, ranges from 0 to 3. The intensity of hormone receptor staining was classified into 3 categories: weak expression level is defined as—0 < ER/PR ≤ 1, intermediate—1 < ER/PR ≤ 2 and strong—ER/PR > 2.
Data on endogenous estrogen exposure and parity were collected including: age of menarche, number of childbirth and age of first delivery, menopausal status and age of menopause. Data on any loco-regional, distant recurrence and death were also recorded. Data lock was in 18/06/2020.Time of recurrence was defined as the date of biopsy from site of recurrence or the date of abnormal imaging suggestive for metastatic disease. Disease-free survival (DFS) was defined as the time between breast surgery to an event (recurrence or death) or data lock. Duration of survival was defined as time from initial breast cancer diagnosis to date of death or time of data lock. Patients’ vital status was ascertained through Israel’s ministry of interior database.
The impact of endogenous estrogen exposure and parity on breast cancer characteristics and on outcomes was evaluated. Comparisons were conducted for the pre-specified subgroups including: age of menarche (age < 12 compared to age ≥ 12), nulliparity (yes compared to no), multiparity (women with 5 or more childbirths compared to less than 5 childbirths) and menopause (postmenopausal compared to premenopausal). For postmenopausal women comparisons were done for early menopause (age < 45 compared to menopause age ≥ 45) and late menopause (age > 55 compared to menopause at the age ≤ 55). This discrimination was applied based to prior data suggesting differences in breast cancer risks between these subgroups [22]. As data were analyzed anonymously, no consent was required. The study protocol was approved by our institutional ethics committee.
2.1 Statistical analysis
Statistical analysis was generated using SAS Software, Version 9.4. Data were reported descriptively by each of the pre-specified categories as described above. Categorical variables were presented by as proportions and continuous variables were presented by mean and standard deviation (SD) or Median (Range) as appropriate. T-test for normally distributed variables or Mann Whitney for non normal were used to compare the value of continuous variables between study groups. Fisher’s exact test was used to compare the value of categorical variables between study groups.
The impact of age of menarche, menopause, age of menopause and parity on DFS and OS were assessed. Overall-survival (OS) and DFS were assessed by Kaplan–Meier survival analysis, with the log-rank test. For significant differences in outcomes in univariate analyses, multivariate analysis for age, tumor size, nodal status, grade and Oncotype RS were assessed by the Cox proportional hazards model. Two-sided p-values less than 0.05 were considered statistically significant.
3 Results
We identified 705 patients with early breast cancer whose tumor were sent to Oncotype DX analysis at our institution from 2005 to 2012. 85 patients were excluded, remaining 620 women who were included in the study cohort.
Median age was 61 years (range 34–85 years), 75% (464) women were postmenopausal, most of them 86% menopaused before the age of 55 and 14% had menopause before the age of 45. Median age of menarche was 13 years and in 86% age of menarche was 12 and above. Median number of deliveries was 3 (range 0–14), 6% were multiparous and 10% were nullipara. Invasive ductal carcinoma (IDC) was the most common histology (81%), followed by invasive lobular carcinoma (ILC) (12%). Women were most likely to present with tumor size ≤ 2 cm (77%) and node negative disease (82%). Grade was well, moderately and poorly differentiated in 17%, 66% and 17% of tumors, respectively with strong ER intensity in 76% of women and negative PR in 14% of women. Ki67 was 20% or lower in 78% of tumors. Oncotype DX RS was 25 or lower in 82%. Perineural and angiolymphatic invasion were uncommon presenting in 9% and 6% of tumors, respectively. Overall, 77% of women with high genomic risk (RS > 25) were treated with adjuvant chemotherapy. 97.7% women were treated with adjuvant endocrine therapy. The initial endocrine therapy was tamoxifen for 87% and aromatase inhibitors for 10.7%. The administration of aromatase inhibitors was more common in postmenopausal women compared to pre-menopausal women (12.5% vs. 3.2%). In the other evaluated subgroups, the distribution of the initial endocrine therapy was similar, see Supplementary Table 1.
Histo-pathological characteristics by the pre-specified subgroups are presented in Table 1. Post-menopausal women were more likely to have stronger ER intensity (79% vs. 65%, p = 0.002), but PR negativity was significantly more frequent (16% vs. 7%, p = 0.009). Early age of menarche was associated with PR positivity (94% vs. 85%, p = 0.039). No other associations were found between endogenous estrogen exposures and tumor characteristics.
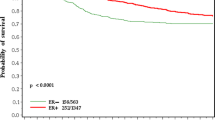
The median duration of follow-up was 10.4 years. The results of DFS are presented in Table 2 and Fig. 1. Estimated 10-year DFS was significantly worse for women with early menopause (71.3% vs. 86.3%), HR = 2.26, 95% CI 1.30–3.93, p = 0.004) and for multiparity (73.9% vs. 85.6%), HR = 2.16, 95% 1.10–4.25, p = 0.026. On multivariate analysis worse DFS remained to be significantly worse for women with early menopause and multiparous women (p = 0.004 and 0.01, respectively) together with oncotype RS (p < 0.001), see Table 3. Compared to premenopausal women, postmenopausal women had worse 10-year DFS that approached significance (83.8% vs. 90.1%, HR = 1.69, 95% 0.92–3.09, p = 0.09). This difference did not remain significant on multivariate analysis (p = 0.289). DFS was comparable in the other evaluated subgroups.
Disease free survival probabilities in a menopausal women, b early menopause (menopause before the age of 45), c late menopause (menopause older the age of 55), d early age at menarche (menarch before the age of 12), e nullipara, f number of deliveries (0–4 vs. 5 and more), g age at first live birth (30 years and older vs. < 30 years)
The results of OS are presented in Table 2 and Fig. 2. Women with early menopause had worse 10-year OS compared to women with menopause at later age (78.6% vs. 90.4%,), HR = 2.60, 95% CI 1.36–4.95, p = 0.004. In multivariate analysis the association between worse OS and early menopause remained significant (p = 0.011), together with older age (p = 0.011) and higher oncotype RS (p = 0.005). Compared to premenopausal women estimated 10-year overall survival was significantly worse in post-menopausal women (88.5% vs. 95.8%), HR = 2.62, 95% CI 1.08–6.38, p = 0.034. In multivariate analyses the menopausal status did not remain associated with worse OS (p = 0.553), Oncotype RS was the only variable that was associated with worse OS (p < 0.001), and the impact of older age on worse OS approached significance (p = 0.063). Results of the multivariate analyses are shown in Table 3. OS was comparable in the other evaluated subgroups. Of note, administration of chemotherapy for women in high genomic risk was significantly lower in post-menopausal women comparted to pre-menopausal women (70% vs. 96%, p = 0.006), but for early menopause and multipara women, rates of chemotherapy administration were comparable to their control.
Overall survival probabilities in a menopausal women, b early menopause (menopause before the age of 45), c late menopause (menopause older the age of 55), d early age at menarche (menarche before the age of 12), e nullipara f number of deliveries (0–4 vs. 5 and more), g age at first live birth (30 years and older vs. < 30 years)
4 Discussion
Lifetime exposure to estrogen that is related to reproductive factors including early menarche, late menopause and parity have a well-established impact on breast cancer risk [2]. While the increased risk is more prominent for ER positive disease rather than ER negative disease, few studies address the effect of reproductive factors on other tumor characteristics and outcomes. Here, we investigated the influence of endogenous estrogen exposure on early-stage luminal breast cancer characteristics including also the impact on genomic risk. Overall, the impact of the evaluated endogenous exposures on breast cancer characteristics was relatively limited. Menopause was associated with stronger ER intensity, but with PR negativity. Women with early menarche were more likely to have PR positive disease. Oncotype RS, a well validated prognostic and predictive factor that is not a subject for inter-laboratory heterogeneity [19], was comparable in all of the evaluated subgroups. No other associations between parity, age of first delivery and age of menopause with tumor characteristics were identified.
Data suggest possible differential effect of reproductive risk factors and obesity according to breast cancer subtype, with parity, age at menarche, age at first birth, breastfeeding and obesity demonstrating stronger associations with luminal subtype compared to other subtypes [17, 20], however data on the effect of these factors of the intensity of ER and PR staining are scarce. In our analysis an association between early menarche and PR positivity was identified. This is in line with other studies showing a higher concordance of ER/PR positive breast cancer in women with early age of menarche [20, 23, 24].
In this study, we reported on the influence of endogenous estrogen exposures on outcome with a follow-up duration of more than 10 years. Interestingly, despite comparable tumor characteristics, early menopause and multiparity were each independently associated with worse outcomes, and these differences remained significant in multivariate analysis. Postmenopausal women also had worse OS compared to premenopausal women, but this difference did not remain significant in multivariate analysis and can probably be attributed to non-breast cancer mortality in older population.
Our finding of adverse outcomes of women who menopaused before the age of 45 is meaningful and might have important implication of treatment decisions. Menopause gives rise to cardiovascular morbidity and metabolic syndrome [25, 26]. Earlier age of menopause enhances the risk for cardiovascular morbidity and mortality [27,28,29]. Given the excellent prognosis of women with luminal early breast cancer, the most common cause of death in this population is cardiovascular disease [30, 31]. Adjuvant treatment for breast cancer might increase cardiovascular morbidity. Anthracycline based adjuvant chemotherapy that are often recommended to women with high genomic risk or to women with high clinical risk [18, 32], might cause cardiotoxicity. Adjuvant treatment with aromatase inhibitors, which are consider the standard of care in all postmenopausal women [33] and can often be considered for higher risk premenopausal in combination with ovarian function suppression [34], have increased odds for cardiovascular morbidity compared to both tamoxifen or placebo [35, 36]. Adjuvant radiation that is given to the vast majority of women after breast conserving surgery, is also associated with cardiovascular toxicity when treating left sided disease [37]. Tailoring treatment decisions based on the individual risk of breast cancer recurrence and on other comorbidities including risk of cardiovascular disease are required. Modifications to reduce treatment related cardiovascular toxicity, such as anthracyclines sparing chemotherapy [38], shorter treatment with AIs [35] and omission of radiation or partial breast irradiation [39, 40] should be considered. Our findings demonstrated that age of menopause might also be an important factor when weighing the risk and benefit balance of treatment options.
Multiparity was also associated with worse DFS. This finding is significant and is in line with previous publications [41, 42], however due to the low number of multiparous women, the conclusions that can be drawn are limited. Multiparity might be associated with low socioeconomic status [43, 44], and hence lower adherence to treatment and higher risk of comorbidities [45]. Multiparty is also an independent predictor for obesity [46, 47], which may also explain worse outcome after breast cancer diagnosis, both due inferior specific breast cancer survival [48] and non-breast cancer mortality [49]. These hypotheses might partially explain our finding, however, our data on weight and socioeconomic status were insufficient. Larger scales studies are needed to elucidate the effect on multiparity on breast cancer outcome.
We acknowledge the limitations of a single-center retrospective cohort with inherent biases. The histopathological variables were not re-evaluated for the study by central pathology. While data on adjuvant chemotherapy and on the initial adjuvant endocrine were collected, data on the duration of therapy, subsequent endocrine therapy (i.e., switch from tamoxifen to aromatase inhibitors) and the addition of ovarian function suppression for premenopausal were not available. Additionally, data on body mass index, an important variable that may affect breast cancer incidence and features [50], were incomplete and therefore were not addressed in this study. On the other hand, considering the scarcity of data on the effect of endogenous estrogen on breast cancer characteristics our cohort represents a large number of patients with long duration of follow up. The effect on all known prognostic pathological characteristics were meticulously collected and the assessment of endogenous estrogen impact on Oncotype DX score is novel.
In conclusion, our results suggest little impact of endogenous estrogen exposure on breast cancer characteristics of early stage, luminal disease. Early menopause and multiparity were associated with worse outcome. These findings might be related to other comorbidities or direct influence on breast cancer pathogenesis. Given the impact of early menopause and multiparty on outcome, these variables should be taken into consideration in treatment decisions on early-stage luminal breast cancer.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. https://doi.org/10.3322/caac.21590.
Key T, Appleby P, Barnes I, Reeves G, Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–16. https://doi.org/10.1093/jnci/94.8.606.
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. https://doi.org/10.1093/jnci/dju055.
Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–51. https://doi.org/10.1016/s1470-2045(12)70425-4.
Ritte R, Lukanova A, Tjønneland A, Olsen A, Overvad K, Mesrine S, et al. Height, age at menarche and risk of hormone receptor-positive and -negative breast cancer: a cohort study. Int J Cancer. 2013;132(11):2619–29. https://doi.org/10.1002/ijc.27913.
Nichols HB, Schoemaker MJ, Cai J, Xu J, Wright LB, Brook MN, et al. Breast cancer risk after recent childbirth: a pooled analysis of 15 prospective studies. Ann Intern Med. 2019;170(1):22–30. https://doi.org/10.7326/m18-1323.
Albrektsen G, Heuch I, Hansen S, Kvåle G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92(1):167–75. https://doi.org/10.1038/sj.bjc.6602302.
Rosner B, Colditz GA, Willett WC. Reproductive risk factors in a prospective study of breast cancer: the Nurses’ Health Study. Am J Epidemiol. 1994;139(8):819–35. https://doi.org/10.1093/oxfordjournals.aje.a117079.
Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Endogenous Hormones Breast Cancer Collaborative Group, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–26. https://doi.org/10.1093/jnci/djg022.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8. https://doi.org/10.1056/nejmsr1606602.
Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–65. https://doi.org/10.1093/jnci/djh336.
Farhat GN, Cummings SR, Chlebowski RT, Parimi N, Cauley JA, Rohan TE, et al. Sex hormone levels and risks of estrogen receptor-negative and estrogen receptor-positive breast cancers. J Natl Cancer Inst. 2011;103(7):562–70. https://doi.org/10.1093/jnci/djr031.
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. https://doi.org/10.1001/jama.2018.19323.
Sparano JA, Crager MR, Tang G, Gray RJ, Stemmer SM, Shak S. Development and validation of a tool integrating the 21-gene recurrence score and clinical-pathological features to individualize prognosis and prediction of chemotherapy benefit in early breast cancer. J Clin Oncol. 2020;39(6):557–64. https://doi.org/10.1200/JCO.20.03007.
Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, et al. ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139(2):539–52.
Clark GM, McGuire WL, Hubay CA, Pearson OH, Marshall JS. Progesterone receptors as a prognostic factor in stage II breast cancer. N Engl J Med. 1983;309(22):1343–7. https://doi.org/10.1056/nejm198312013092240.
Lambertini M, Santoro L, Del Mastro L, Nguyen B, Livraghi L, Ugolini D, et al. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev. 2016;49:65–76. https://doi.org/10.1016/j.ctrv.2016.07.006.
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–21. https://doi.org/10.1056/nejmoa1804710.
Curtit E, Mansi L, Maisonnette-Escot Y, Sautière JL, Pivot X. Prognostic and predictive indicators in early-stage breast cancer and the role of genomic profiling: focus on the Oncotype DX® Breast Recurrence Score Assay. Eur J Surg Oncol. 2017;43(5):921–30. https://doi.org/10.1016/j.ejso.2016.11.016.
Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250–63. https://doi.org/10.1093/jnci/djq526.
Weidner N, Cote RJ, Suster S, et al. Modern surgical pathology. 2nd ed. Philadelphia: Saunders Elsevier; 2009.
Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–85. https://doi.org/10.1056/nejm200101253440407.
Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, Moorman PG. Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol. 2000;151(7):703–14. https://doi.org/10.1093/oxfordjournals.aje.a010265.
Yoo KY, Tajima K, Miura S, Takeuchi T, Hirose K, Risch H, et al. Breast cancer risk factors according to combined estrogen and progesterone receptor status: a case-control analysis. Am J Epidemiol. 1997;146(4):307–14. https://doi.org/10.1093/oxfordjournals.aje.a009271.
Coyoy A, Guerra-Araiza C, Camacho-Arroyo I. Metabolism regulation by estrogens and their receptors in the central nervous system before and after menopause. Horm Metab Res. 2016;48(8):489–96.
Morselli E, Santos RS, Criollo A, Nelson MD, Palmer BF, Clegg DJ. The effects of oestrogens and their receptors on cardiometabolic health. Nat Rev Endocrinol. 2017;13(6):352–64.
Yang L, Lin L, Kartsonaki C, Guo Y, Chen Y, Bian Z, et al. Menopause characteristics, total reproductive years, and risk of cardiovascular disease among chinese women. Circ Cardiovasc Qual Outcomes. 2017;10(11): e004235. https://doi.org/10.1161/CIRCOUTCOMES.117.004235.
Ley SH, Li Y, Tobias DK, Manson JE, Rosner B, Hu FB, et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. 2017;6(11): e006713. https://doi.org/10.1161/JAHA.117.006713.
Peters SA, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104(13):1069–75. https://doi.org/10.1136/heartjnl-2017-312289.
Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64.
Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–92.
Cardoso F, van Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717–29. https://doi.org/10.1056/nejmoa1602253.
Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, DeCensi A, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37(33):3152–65. https://doi.org/10.1200/jco.19.01472.
Francis PA, Regan MM, Fleming GF, Láng I, Ciruelos E, Bellet M, International Breast Cancer Study Group, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–46. https://doi.org/10.1056/nejmoa1412379.
Goldvaser H, Barnes TA, Šeruga B, Cescon DW, Ocaña A, Ribnikar D, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(1):31–9. https://doi.org/10.1093/jnci/djx141.
Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299–309. https://doi.org/10.1093/jnci/djr242.
Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98.
Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE Jr, Jacobs SA, et al. Anthracyclines in early breast cancer: the ABC Trials-USOR 06–090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol. 2017;35(23):2647–55. https://doi.org/10.1200/JCO.2016.71.4147.
Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM, PRIME II investigators, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–73. https://doi.org/10.1016/s1470-2045(14)71221-5.
Korzets Y, Fyles A, Shepshelovich D, Amir E, Goldvaser H. Toxicity and clinical outcomes of partial breast irradiation compared to whole breast irradiation for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;175(3):531–45. https://doi.org/10.1007/s10549-019-05209-9.
Sun X, Nichols HB, Tse CK, Bell MB, Robinson WR, Sherman ME, et al. Association of parity and time since last birth with breast cancer prognosis by intrinsic subtype. Cancer Epidemiol Biomark Prev. 2016;25(1):60–7. https://doi.org/10.1158/1055-9965.epi-15-0864.
Jääskeläinen A, Roininen N, Karihtala P, Jukkola A. High parity predicts poor outcomes in patients with luminal B-Like (HER2 Negative) early breast cancer: a prospective Finnish Single-Center Study. Front Oncol. 2020;10:1470. https://doi.org/10.3389/fonc.2020.01470.
Kutty VR, Thankappan KR, Kannan KP, Aravindan KP. How socioeconomic status affects birth and death rates in rural Kerala, India: results of a health study. Int J Health Serv. 1993;23(2):373–86. https://doi.org/10.2190/9N4P-F1L2-13HM-CQVW.
Shawky S, Rashad H, Khadr Z. Reproductive health inequalities in Egypt. Evidence for guiding policies, final report: UNFPA/ASRO. 2018.
Schultz WM, Kelli HM, Varghese T, Shen J, Sandesara P, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137(20):2166–78. https://doi.org/10.1161/CIRCULATIONAHA.117.029652.
Heliövaara M, Aromaa A. Parity and obesity. J Epidemiol Community Health. 1981;35(3):197–9. https://doi.org/10.1136/jech.35.3.197.
Hajiahmadi M, Shafi H, Delavar MA. Impact of parity on obesity: a cross-sectional study in Iranian women. Med Princ Pract. 2015n;24(1):70–4. https://doi.org/10.1159/000368358.
Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat. 2012;134(2):769–81. https://doi.org/10.1007/s10549-012-2073-x.
Dignam JJ, Wieand K, Johnson KA, Raich P, Anderson SJ, Somkin C, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97(3):245–54. https://doi.org/10.1007/s10549-005-9118-3.
Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, O’Brien KM, Premenopausal Breast Cancer Collaborative Group, et al. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018;4(11):e181771. https://doi.org/10.1001/jamaoncol.2018.1771.
Funding
None for all authors.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data and participated in drafting the article or revising it critically for important intellectual content and gave final approval of the version to be submitted and any revised version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures in the study were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical Review Board: Rabin medical center institutional ethics committee, reference number: 0576-19-RMC. As data were analyzed anonymously, no informed consent was required.
Competing interests
Dr. Korzets reports speaker honorarium from Roche, outside of submitted work. Dr. Moore reports speaker honorarium from Roche and Merck, all outside the submitted work. Prof. Yerushalmi reports personal fees from: Roche (Consulting, invited speaker), Pfizer (Consulting), Novartis (Consulting), Teva (Invited speaker), Medison (Invited speaker), MSD (Invited speaker), Astra-Zeneca (Invited speaker) and Novartis (Invited speaker), all outside the submitted work. Dr. Goldvaser reports personal fees from: Roche (honorarium), Pfizer (honorarium), Novartis (honorarium and consulting) and Oncotest (honorarium) all outside the submitted work. All other authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Korzets, Y., Yariv, O., Mutai, R. et al. The impact of endogenous estrogen exposures on the characteristics and outcomes of estrogen receptor positive, early breast cancer. Discov Onc 12, 26 (2021). https://doi.org/10.1007/s12672-021-00420-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-021-00420-x