Abstract
Hyperglycemia may lead to proliferation, invasion, apoptosis inhibition, migration, and eventually metastasis of cancer cells by several mechanisms. In this study, the effect of hyperglycemia on overall survival (OS), disease-free survival (DFS), and locoregional recurrence (LRR) was investigated in NSCLC. One stage IIIA-IIIB NSCLC patient treated with chemoradiotherapy between 2010 and 2015 was enrolled. Fasting blood glucose (FBG) levels were recorded in pre-treatment, treatment, and post-treatment periods. Median age was 54 years (51–62). Fifty-two patients had squamous cell carcinoma (SCC); 19 had adenocarcinoma. Median follow-up was 19 (11–30), median survival was 19 (13–24), and DFS was 9 (7–11) months. Diabetic patients had shorter survival than non-diabetics 12 (95%CI, 10–14) vs. 25 months (95%CI,18–32), p = 0.005. Number of patients with LRR was also higher in diabetics compared to non-diabetics (8/12 vs. 11/37, p = 0.039). OS was shorter in patients with hyperglycemic-FBG and diabetic-FBG levels in pre-treatment period (log-rank p = 0.03 and 0.023, respectively). Diabetic-FBG level in pre-treatment period was found to be the only independent risk factor for survival. In subgroup analysis, these differences were apparent in SCC (log-rank p = 0.009 for hyperglicemic, log-rank p = 0.017 for diabetic-FBG). LRR was 68% in patients with diabetic-FBG, 36.5% in patients with non-diabetic-FBG in post-treatment period (p = 0.015). Patients with LRR had significantly higher median FBG value in post-treatment period compared to non-relapsing patients, 138 mg/dL (119–228) and 111 mg/dL (99–164), respectively (p = 0.022). The patients with hyperglycemic and diabetic-FBG levels in pre-treatment period had shorter survival compared to normoglycemic ones. The patients with diabetic-FBG level in post-treatment period had higher LRR, and relapsing patients had higher FBG levels in post-treatment period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increased cancer risk, including lung cancer, is shown in diabetic patients by epidemiological studies [1,2,3]. But the data about the effect of diabetes on cancer outcome has conflicting results [4] changing from poorer [5,6,7,8] to improved outcomes [9,10,11]. The direct (e.g., due to hyperglycemia) or indirect effects (e.g., due to hyperinsulinemia, increased IGF-1 in tumor microenviroment resulting in cancer cell proliferation and metastasis) [7, 12], the possibility of less intensive treatment approaches for diabetic cancer patients and higher complication risks [13] are the possible theories underlying the poorer outcomes. The opposite theory which advocates improved outcome claims that diabetes impedes cancer cell invasion through basocellular membrane by diabetic microangiopathy through microvascular changes which might result in decreased metastatic ability and improved prognosis [9].
Non-small cell lung carcinoma (NSCLC) is the most common solid tumor [14] and is the leading cause of death among cancer patients worldwide [15]. Eight to 18% of newly diagnosed cancer patients have diabetes, and until now, there has been a debate concerning the influence of diabetes on lung cancer prognosis [4]. Thus, the prognostic significance of diabetes is still unknown in NSCLC [16, 17].
“Warburg effect” defines the use of glucose by glycolysis instead of mitochondrial oxidative phosphorylation regardless of oxygen status which is a specific mechanism for cancer cells and confers an advantage over normal cells [18]. Furthermore, cancer cells accumulate and use elevated amounts of glucose relative to normal counterparts to be able to replace increased demand for carbon which is used in anabolic reactions [19]. Therefore, cancer cells upregulate their glucose transporters (Glut-1), and their survival seems to be strictly dependent on the glucose concentration changes [20]. Glucose deprivation leads to growth retardation and death of cancer cells [21]. Recently, glucose transport inhibitors have been investigated as novel anticancer agents, both in vivo and in vitro [22]. Contrary to this, continuous exposure to hyperglycemia provides high-glucose fuel source to cancer cells. This may promote carcinogenesis by cancer cell proliferation, apoptosis inhibition, invasion, migration, and eventually metastasis through several mechanisms—PI3K/Akt stimulated by insulin and IGF-1 receptors, RAS/MAP kinase pathways [12, 16, 23]. Also, hyperglycemia may induce oxidative stress and reactive oxygen species (ROS) production by mitochondrial dysfunction [16, 24]. Decreased mitochondrial function switches the cell energy metabolism from oxidative phosphorylation to glycolysis [25]. Besides, it may stabilize hypoxia-inducible factor-1 alpha (HIF-1α) which is mandatory for expression of glycolytic enzymes [26]. In addition to this, hyperglycemia may result in many epigenetic changes (e.g., histone acetylation, NF-ƙB activation, IL-6, and TNF-α release) [27, 28], may promote expansion of tumor-initiating bronchoalveolar stem cells (BASCs), and may induce autonomous hyperplasia of BASCs [29].
The high rate of glucose consumption by tumor cells can easily be visualized by 18Fluorodeoxyglucose positron emission tomography (18FDG-PET) in clinic [30]. Also, fasting glucose levels ≥ 100 mg/dl found to be associated with excess risk of death in cancer patients [7]. All of these data might warn us that; hyperglycemia might promote tumor activity.
In this regard, the objective of this study is to investigate the effect of fasting blood glucose (FBG) levels in pre-treatment, treatment, and post-treatment periods on loco-regional recurrence (LRR), disease-free survival (DFS), and overall survival (OS) in stage III NSCLC patients treated with concurrent chemoradiotherapy (CRT).
Methods
Stage III NSCLC patients who received concurrent CRT between 2010 and 2015 were enrolled. Patients’ data derived from hospital records were evaluated retrospectively, in terms of patient characteristics, ECOG performance status, weight loss, histological subtype, tumor stage, treatment characteristics, history of diabetes, and FBG levels at pre-treatment, treatment, and post-treatment periods. Treatment outcomes, locoregional recurrence (LRR), disease-free survival (DFS), and overall survival (OS), were reviewed and reported based on glycemic status. The LRR and distant metastases (DM) were diagnosed by computed tomography or PET/BT. LRR was described as tumor regrowth at the primary or regional lymph nodes. DFS was calculated from the last day of CRT until LRR or DM. OS was measured from the date of diagnosis to date of death. OS data were collected from national database, therefore were available for all patients. Patients treated with sequential chemotherapy (CT)-RT or surgery after neoadjuvant CRT were excluded to avoid heterogeneity in patient treatment and clinical characteristics to be able to determine FBG as the main prognostic factor for the study of interest.
Fasting was defined as no caloric intake for at least 8 h. FBG levels were recorded at three different time periods: (i) pre-treatment (between the diagnosis and the start of CRT), (ii) treatment (during CRT), and (iii) post-treatment (between the end of CRT/consolidative CT and the first recurrence (local/distant) or death in case of no recurrence). In patients with multiple FBG levels for each time period, the highest value for that time period was taken into account.
FBG levels were categorized as normal if FBG is < 110 mg/dl, as hyperglicemic if FBG is ≥ 110 mg/dl, as diabetic if FBG is ≥ 126 mg/dl, and as non-diabetic if FBG is < 126 mg/dl [31, 32].
Statistical Analysis
Continuous variables were expressed as median (25–75 percentile) and categorical variables as numbers (%). LRR, DFS, and OS were analyzed using the Kaplan-Meier method. After univariate analysis, factors that might have an impact on survival were analyzed by Cox regression model in multivariate analysis. p values were derived from two-tailed tests, and p < 0.05 was considered as statistically significant.
Results
A total of 71 NSCLC patients who received concurrent chemoradiotherapy between 2010 and 2015 and who had at least one FBG data in each three time periods were eligible for inclusion. The median follow-up time was 19 (11–30) months. All of the patients had ECOG performance status 0–1 and weight loss less than 10% in last 6 months. Median age at diagnosis was 54 years (51–62). Sixty-four patients (90%) were male. Fifty-two patients (73%) had SCC, 19 (27%) had adenocarcinoma, 58% was in Stage IIIA, and 42% was in stage IIIB. All patients received 3-D conformal RT; median dose was 63 Gy (63–63). Type of concurrent CT was cisplatin 75 mg/m2 D1 and etoposide 100 mg/m2 D1–3 every 4 weeks, median number of concurrent CT cycles was 2 (2–2). Twenty patients (28%) received consolidative CT with the same schedule but administered in every 3 weeks.
Three-year survival rate was 18% and median survival was 19 months (95% CI, 13–24). DFS was 9 months (95% CI, 7–11). Twenty-eight cases (40%) had LRR, and it was non-significantly higher in the SCC patients.
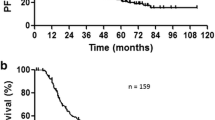
A previous history of diabetes was recorded in 12 (16.9%) patients. Information about medical treatment of diabetes was missing in all but in one patient, and it was metformin. Diabetic patients had higher LRR (8/12 vs. 11/37, p = 0.039) and lower OS than non-diabetics; 12 months (95% CI, 10–14) vs. 25 months (95% CI, 18–32), p = 0.005 (Fig. 1).
Among patients without previous history of diabetes (59 patients), 11.8, 20.3, and 23.7% had hyperglicemic and 20.3, 45.7, and 40.6% had diabetic-FBG levels in pre-treatment, treatment, and post-treatment periods, respectively.
Proportion of patients with hyperglycemic and diabetic-FBG levels according to treatment periods are shown in Table 1.
Median FBG levels in all patients, in patients with LRR and in patients without LRR, in pre-treatment, treatment, and post-treatment periods are shown in Table 2.
Factors that were considered to have an impact on survival such as age, gender, LRR, histologic subtype, and pre-treatment, treatment, and post-treatment FBG levels were introduced into a multivariate Cox regression model to evaluate the independent risk factors for survival. Diabetic-FBG level in pre-treatment period was found to be the only independent risk factor for survival (HR, 2.02; CI 95%, 1.03–3.95; p 0.039).
The Association Between FBG Levels in Pre-Treatment Period and Treatment Outcomes
OS was lower in hyperglicemic and diabetic patients (log-rank p = 0.03 and 0.023, respectively) (Fig. 2). In the subgroup analysis, these differences were apparent in the SCC subtype (log-rank p = 0.009 for hyperglicemic and log-rank p = 0.017 for diabetic patients (Fig. 3).
Kaplan-Meier survival of all patients according to fasting blood glucose (FBG) levels in pre-treatment period. a Median survival was 23 (%95 CI, 16–29) months in normal (FBG < 110 mg/dl) and 12 (%95 CI, 8–14) months in hyperglicemic (FBG ≥ 110)patients. b Median survival was 21 (%95 CI, 15–26) months in non-diabetic (FBG < 126 mg/dl) and 12 (%95 CI, 9–15) months in diabetic (FBG ≥ 126 mg/dl) patients
Kaplan-Meier survival of SCC patients according to fasting blood glucose (FBG) levels in pre-treatment period. a Median survival was 27 (%95 CI, 21–33) months in normal (FBG < 110) and 13 (%95 CI, 9–17) months in hyperglicemic (FBG ≥ 110) patients. b Median survival was 26 (%95 CI. 21–31) months in non-diabetic (FBG < 126 mg/dl) and 12 (%95 CI, 9–15) months in diabetic (FBG ≥ 126) patients
The Association Between FBG Levels in Treatment Period and Treatment Outcomes
Patients with higher median FBG of 135 mg/dL (111–234) had non-significant trend for LRR compared to patients with lower median FBG of 128 mg/dL (102–157) (p = 0.24). The median FBG levels were 135 mg/dL (106–191), and114 mg/dL (96–142) in SCC and adenocarcinoma patients, respectively, (p = 0.025).
The Association Between FBG Levels in Post-Treatment Period and Treatment Outcomes
LRR was 19/28 (68%) in patients with diabetic FBG, and it was 15/41 (36.5%) in patients with non-diabetic FBG (p = 0.015).
The Association Between Median FBG Levels and Treatment Outcomes
Patients with LRR had significantly higher median FBG level in post-treatment period compared to non-relapsing patients, 138 mg/dL (119–228) and 111 mg/dL (99–164), respectively, (p = 0.022) (Table 2).
Also, FBG levels of all individual patients according to treatment periods are shown in Fig. 4.
Discussion
In our study, we identified that patients who had hyperglycemic or diabetic-FBG levels in pre-treatment period had significantly worse survival. The patients with diabetic-FBG level in post-treatment period had higher LRR. Also, relapsing patients had higher median FBG in post-treatment period. Therefore, hyperglycemia in pre-treatment period may provide information regarding survival, and diabetic-FBG level may be an independent risk factor for survival. Besides, diabetic-FBG levels in post-treatment period may pose a risk for LRR.
Strict selection criteria for concurrent CRT resulted in homogenous cohort of patients composed of only stage III, relatively younger aged, ECOG 0–1, weight loss less than 10% in last 6 months and treated with concurrent CRT. Besides these, exclusion of patients treated with sequential CT-RT or surgery after neoadjuvant treatment might enable us to exclude effects of main prognostic factors (stage, ECOG, weight loss, treatment type) on prognosis and to analyze FBG as a main prognostic factor. Also, relatively younger age of our patient population gave us an opportunity to have a cohort with relatively lower incidence of co-morbidities, since it is known that 60% of newly diagnosed cancer patients older than 65 years have serious co-morbidity [13].
In our study, the prevalence of patient reported diabetes in NSCLC patients was almost four times higher than normal population [13, 17], in concordance with the knowledge of higher prevalence of cancer in diabetic patients [1,2,3]. Similarly, the prevalence was 18.9% among NSCLC patients in the study by Imai et al. [16]. Although the data about the effect of diabetes on cancer outcome has conflicting results [4], our findings about lower OS and higher LRR in NSCLC patients with history of self-reported diabetes compared to non-diabetics in concordance with literature [5,6,7,8].
Recently, high blood glucose level has been found to be associated with poor survival in many types of cancer [33,34,35,36,37,38,39]. But, there is very limited besides conflicting data in lung cancer patients [7, 11, 40,41,42,43,44]. Also, most of these studies included mainly metastatic patients, patients with various types of cancer treatments [40,41,42,43,44], and only glucose levels at the time of diagnosis were taken into account. To our knowledge, our study is the first one considering FBG levels in treatment and post-treatment periods as well as at the time of diagnosis (pre-treatment) as a novel prognostic factor in stage III NSCLC patients treated with CRT.
Since FBG level is the main diagnostic tool for diabetes [32], we investigated FBG levels instead of self-reported history of diabetes to avoid non- or under-diagnosed diabetes. In a study by Luo, only half of NSCLC patients having FBG levels ≥ 126 mg/dl reported themselves as diabetic [41]. Similarly, in our study, among patients who did not report themselves as diabetic, percentage of patients having hyperglicemic and diabetic FBG were relatively high in all three time periods (Table 1). Identification of higher number of hyperglycemic patients compared to self-reported diabetic ones should draw attention to non-diagnosed or under-diagnosed diabetic cancer patient population. Besides, a number of medical conditions other than diabetes such as obesity [45], pancreatitis [46], chronic stress, or some medications [47] might cause hyperglycemia. All these factors forced us to take FBG levels into consideration for the analysis.
In a study by Imai et al., authors found diabetes (based on medical history and FBG ≥ 126 mg/dl) to be an independent prognostic factor for survival in NSCLC [16] which is similar to our finding about the association between having diabetic-FBG levels in pre-treatment period and lower OS.
To our knowledge, there is only one data, in abstract form, considering impact of hyperglycemia in pre-treatment, treatment, and post-treatment periods in cervical cancer patients treated with radical chemoradiotherapy. It was demonstrated that hyperglycemic patients in post-treatment period had higher LRR which is in concordance with our findings.
Taken together, our results show compelling evidence for having patients in euglycemic state in pre-treatment, post-treatment, and perhaps in treatment periods and pay attention to early detection and correction of hyperglycemia more strictly than before in NSCLC. Also, our data may provide possible rationale for the use of antidiabetics together with anticancer treatment to regulate glycemia. In 2005, metformin, the most widely used drug for type 2 diabetes was introduced as anticancer drug, and it has been the focus of cancer research since then [48]. In addition to its indirect effect by lowering blood glucose levels, it has direct anticancer effects. In an animal study by Storozhuk et al., it was shown that by using metformin along with RT-CT in lung cancer, tumor growth rate might be reduced and radiosensitivity might be enhanced, possibly through ATM–AMPK pathway [49]. Recently, a meta-analysis showed that metformin might be used as an adjuvant agent in cancer treatment [50].
It is known that hyperglycemia may induce autonomous hyperplasia of BASCs through K-Ras activation which is important in initiation of lung adenocarcinoma [29]. In this regard, it was interesting for us to find out that OS was significantly lower and LRR was non-significantly higher in hyperglycemic SCC patients in pre-treatment period compared to adenocarcinomas. The reason for this discrepancy is hard to explain only by significantly higher median FBG levels found in SCC patients in treatment period, but could be the relatively low ratio of adenocarcinoma patient subgroup in our study population.
Retrospective and single-center nature, small sample sizes are the major limitations of our study. Also data on use of metformin in diabetic patients which might have influence on clinical outcome of patients with high FBG levels was missing. In addition, fasting insulin levels, HbA1c, HOMA, and body mass index data were missed. But, the consideration of FBG levels instead of history of diabetes in treatment and post-treatment periods as well as at the time of diagnosis, homogenous cohort of patient can be suggested as the strengths of our study.
Conclusion
Patients with hyperglycemic or diabetic-FBG levels in pre-treatment period may have significantly lower survival. In addition, diabetic-FBG level in pre-treatment period might be an independent factor for survival. Besides, in post-treatment period, patients with diabetic FBG had higher LRR, and relapsing patients had higher median FBG levels. The suggestion that hyperglycemia in pre-treatment and post-treatment periods confers a poor prognostic factor in NSCLC is a novel finding. In spite of underpowered nature of our study, our findings may take attention to glycemic homeostasis and even to consider oral antidiabetic agents in hyperglycemic NSCLC patients. Further, prospective large scale studies are needed to verify the predictive value of FBG on treatment outcomes in NSCLC.
References
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D (2010) Diabetes and cancer: a consensus report. Diabetes Care 33(7):1674–1685. https://doi.org/10.2337/dc10-0666
Satija A, Spiegelman D, Giovannucci E, Hu FB (2015) Type 2 diabetes and risk of cancer. BMJ 350(jan02 1):g7707. https://doi.org/10.1136/bmj.g7707
Larsson SC, Mantzoros CS, Wolk A (2007) Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 121(4):856–862. https://doi.org/10.1002/ijc.22717
Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL (2008) Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 300(23):2754–2764. https://doi.org/10.1001/jama.2008.824
Stein KB, Snyder CF, Barone BB, Yeh HC, Peairs KS, Derr RL, Wolff AC, Brancati FL (2010) Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta-analysis. Dig Dis Sci 55(7):1839–1851. https://doi.org/10.1007/s10620-009-0944-8
Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, Brancati FL, Wolff AC (2011) Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol 29(1):40–46. https://doi.org/10.1200/JCO.2009.27.3011
Seshasai SR et al (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364(9):829–841
LeRoith D, Novosyadlyy R, Gallagher E, Lann D, Vijayakumar A, Yakar S (2008) Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes 116(Suppl 1):S4–S6. https://doi.org/10.1055/s-2008-1081488
Nerlich AG, Hagedorn HG, Böheim M, Schleicher ED (1998) Patients with diabetes-induced microangiopathy show a reduced frequency of carcinomas. In Vivo 12(6):667–670
De Giorgio R, Barbara G, Cecconi A, Corinaldesi R, Mancini AM (2000) Diabetes is associated with longer survival rates in patients with malignant tumors. Arch Intern Med 160(14):2217. https://doi.org/10.1001/archinte.160.14.2217
Hanbali A, al-Khasawneh K, Cole-Johnson C, Divine G, Ali H (2007) Protective effect of diabetes against metastasis in patients with non-small cell lung cancer. Arch Intern Med 167(5):513. https://doi.org/10.1001/archinte.167.5.513-a
Richardson LC, Pollack LA (2005) Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol 2(1):48–53. https://doi.org/10.1038/ncponc0062
Janssen-Heijnen ML et al (2005) Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol 55(3):231–240. https://doi.org/10.1016/j.critrevonc.2005.04.008
Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer Statistics, 2009. CA Cancer J Clin 59(4):225–249. https://doi.org/10.3322/caac.20006
Imai H, Kaira K, Mori K, Ono A, Akamatsu H, Matsumoto S, Taira T, Kenmotsu H, Harada H, Naito T, Murakami H, Endo M, Nakajima T, Yamada M, Takahashi T (2015) Prognostic significance of diabetes mellitus in locally advanced non-small cell lung cancer. BMC Cancer 15(1):989. https://doi.org/10.1186/s12885-015-2012-4
Varlotto J, Medford-Davis LN, Recht A, Flickinger J, Schaefer E, Shelkey J, Lazar M, Campbell D, Nikolov M, DeCamp MM (2012) Confirmation of the role of diabetes in the local recurrence of surgically resected non-small cell lung cancer. Lung Cancer 75(3):381–390. https://doi.org/10.1016/j.lungcan.2011.07.019
Warburg O (1956) On the origin of cancer cells. Science 123(3191):309–314. https://doi.org/10.1126/science.123.3191.309
Dasgupta A, Nomura M, Shuck R, Yustein J (2016) Cancer’s Achilles’ heel: apoptosis and necroptosis to the rescue. Int J Mol Sci 18(1). https://doi.org/10.3390/ijms18010023
Kim JW, Dang CV (2006) Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res 66(18):8927–8930. https://doi.org/10.1158/0008-5472.CAN-06-1501
Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB (2001) Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol 21(17):5899–5912. https://doi.org/10.1128/MCB.21.17.5899-5912.2001
Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X (2012) A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther 11(8):1672–1682. https://doi.org/10.1158/1535-7163.MCT-12-0131
Gallagher EJ, LeRoith D (2011) Diabetes, cancer, and metformin: connections of metabolism and cell proliferation. Ann N Y Acad Sci 1243(1):54–68. https://doi.org/10.1111/j.1749-6632.2011.06285.x
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GRS, Chandel NS (2010) Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 107(19):8788–8793. https://doi.org/10.1073/pnas.1003428107
Agrimi G, Brambilla L, Frascotti G, Pisano I, Porro D, Vai M, Palmieri L (2011) Deletion or overexpression of mitochondrial NAD+ carriers in Saccharomyces cerevisiae alters cellular NAD and ATP contents and affects mitochondrial metabolism and the rate of glycolysis. Appl Environ Microbiol 77(7):2239–2246. https://doi.org/10.1128/AEM.01703-10
Semenza GL (2013) HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123(9):3664–3671. https://doi.org/10.1172/JCI67230
Shyamasundar S, Jadhav SP, Bay BH, Tay SSW, Kumar SD, Rangasamy D, Dheen ST (2013) Analysis of epigenetic factors in mouse embryonic neural stem cells exposed to hyperglycemia. PLoS One 8(6):e65945. https://doi.org/10.1371/journal.pone.0065945
Kim HJ, Kim SH, Yun JM (2012) Fisetin inhibits hyperglycemia-induced proinflammatory cytokine production by epigenetic mechanisms. Evid Based Complement Alternat Med 2012:639469
Micucci C, Orciari S, Catalano A (2014) Hyperglycemia promotes K-Ras-induced lung tumorigenesis through BASCs amplification. PLoS One 9(8):e105550. https://doi.org/10.1371/journal.pone.0105550
Ben-Haim S, Ell P (2009) 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med 50(1):88–99. https://doi.org/10.2967/jnumed.108.054205
Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P, Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (2003) Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26(11):3160–3167
American Diabetes Association (2017) Classification and Diagnosis of Diabetes. Diabetes Care 40, (Suppl 1):S11–S24
Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD, Laughlin GA, Saquib N, Rock CL, Pierce JP (2011) Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol 29(1):54–60. https://doi.org/10.1200/JCO.2010.29.3183
Chu CK, Mazo AE, Goodman M, Egnatashvili V, Sarmiento JM, Staley CA, Galloway JR, Adsay NV, Jacobs S, Kooby DA (2010) Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol 17(2):502–513. https://doi.org/10.1245/s10434-009-0789-6
Kiderlen M, de Glas NA, Bastiaannet E, Engels CC, van de Water W, de Craen AJM, Portielje JEA, van de Velde CJH, Liefers GJ (2013) Diabetes in relation to breast cancer relapse and all-cause mortality in elderly breast cancer patients: a FOCUS study analysis. Ann Oncol 24(12):3011–3016. https://doi.org/10.1093/annonc/mdt367
Nomelini RS, Neto AS, Capuci KA, Murta BM, Murta EF (2011) Relationship between plasma glucose levels and malignant uterine cervical neoplasias. Clin Med Insights Oncol 5:77–82. https://doi.org/10.4137/CMO.S6916
Tieu MT, Lovblom LE, McNamara MG, Mason W, Laperriere N, Millar BA, Ménard C, Kiehl TR, Perkins BA, Chung C (2015) Impact of glycemia on survival of glioblastoma patients treated with radiation and temozolomide. J Neuro-Oncol 124(1):119–126. https://doi.org/10.1007/s11060-015-1815-0
In Choi J, Chang HK, Lee DW, Lee KH, Park JS, Lee HN (2015) Does diabetes mellitus have an impact on the prognosis for patients with cervical cancer? Gynecol Oncol 139(2):319–323. https://doi.org/10.1016/j.ygyno.2015.09.004
Li J, Wu Mf, Lu Hw, Zhang Bz, Wang Lj, Lin Zq (2016) Impact of hyperglycemia on outcomes among patients receiving neoadjuvant chemotherapy for bulky early stage cervical cancer. PLoS One 11(11):e0166612. https://doi.org/10.1371/journal.pone.0166612
Nakazawa K, Kurishima K, Tamura T, Ishikawa H, Satoh H, Hizawa N (2013) Survival difference in NSCLC and SCLC patients with diabetes mellitus according to the first-line therapy. Med Oncol 30(1):367. https://doi.org/10.1007/s12032-012-0367-9
Luo J, Chen YJ, Chang LJ (2012) Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients. Lung Cancer 76(2):242–247. https://doi.org/10.1016/j.lungcan.2011.10.019
Inal A, Kaplan MA, Kucukoner M, Urakcı Z, kılınc F, Isıkdogan A (2014) Is diabetes mellitus a negative prognostic factor for the treatment of advanced non-small-cell lung cancer? Rev Port Pneumol 20(2):62–68. https://doi.org/10.1016/j.rppneu.2013.09.001
Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P (2003) Impact of comorbidity on lung cancer survival. Int J Cancer 103(6):792–802. https://doi.org/10.1002/ijc.10882
Hatlen P, Grønberg BH, Langhammer A, Carlsen SM, Amundsen T (2011) Prolonged survival in patients with lung cancer with diabetes mellitus. J Thorac Oncol 6(11):1810–1817. https://doi.org/10.1097/JTO.0b013e31822a75be
Martyn JA, Kaneki M, Yasuhara S (2008) Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology 109(1):137–148. https://doi.org/10.1097/ALN.0b013e3181799d45
Fogar P, Pasquali C, Basso D, Floreani A, Piva MG, de Paoli M, Melis A, Sperti C, Pedrazzoli S, Plebani M (1998) Transforming growth factor beta, fibrogenesis and hyperglycemia in patients with chronic pancreatitis. J Med 29(5–6):277–287
Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA (2006) Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract 12(4):358–362. https://doi.org/10.4158/EP.12.4.358
Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005) Metformin and reduced risk of cancer in diabetic patients. BMJ 330(7503):1304–1305. https://doi.org/10.1136/bmj.38415.708634.F7
Storozhuk Y, Hopmans SN, Sanli T, Barron C, Tsiani E, Cutz JC, Pond G, Wright J, Singh G, Tsakiridis T (2013) Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer 108(10):2021–2032. https://doi.org/10.1038/bjc.2013.187
Coyle C, Cafferty FH, Vale C, Langley RE (2016) Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol 27(12):2184–2195. https://doi.org/10.1093/annonc/mdw410
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Disclosure Statement
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kirakli, E.K., Yilmaz, U., Yilmaz, H. et al. Fasting Blood Glucose Level in Locally Advanced Non-Small Cell Lung Cancer: a New Prognostic Factor?. HORM CANC 9, 188–196 (2018). https://doi.org/10.1007/s12672-018-0322-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-018-0322-0