Abstract
Objectives
Mental health problems are highly prevalent in people with Alzheimer’s disease (AD) and their family caregivers. Therefore, this study aimed to examine the effects of the Mindfulness-Based Health Care Program (MBHC) on the psychological distress of family caregivers of people with AD and, indirectly, on the behavioral and psychological symptoms of dementia (BPSD) in people with AD.
Method
A sample of 66 caregivers of people with AD was randomly assigned to either 8 weeks of MBHC or treatment as usual (TAU) groups. The psychological distress in family caregivers, measured by the Hospital Anxiety and Depression Scale (HADS), and BPSD in patients, measured by the Neuropsychiatric Inventory Questionnaire (NPI-Q), were evaluated and compared at baseline, post-intervention, and 3-month follow-up.
Results
A total of 50 participants (75.6%) completed the MBHC post-intervention and 30 (50%) at the 3-month follow-up. At post-intervention, compared to TAU, MBHC was associated with significantly greater decreases in psychological distress (β = − 3.86; 95%CI, − 7.67, − 0.04; p = 0.047), specifically in anxiety symptoms (β = − 2.84; 95%CI, − 5.38, − 0.30; p = 0.029), but no significant changes were observed in depressive symptoms. MBHC did not produce a significant change in psychological distress at 3-month follow-up. MBHC did not yield a significant reduction in BPSD in people with AD, neither at post-intervention nor at 3-month follow-up.
Conclusions
The results suggest that 8-week mindfulness training can effectively reduce psychological distress and anxiety symptoms in caregivers of people with Alzheimer’s disease.
Preregistration
This study was preregistered on ClinicalTrials.gov (identifier NCT03858283) on 26 February 2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Alzheimer’s disease (AD) is the cause of approximately 60–70% of dementia cases (Gauthier et al., 2022; World Health Organization, 2021) and its prevalence in Spain is estimated at 6.8% (Niu et al., 2017). Clinically, it is characterized by an insidious and progressive deterioration of cognitive functions and by the presence of behavioral and psychological symptoms of dementia (BPSD) (Chen et al., 2021; Olazaran-Rodriguez et al., 2012).
The prevalence of BPSD in AD is high. More than 90% of people with AD will suffer at least one BPSD during the course of the disease (Preuss et al., 2016). The most frequent BPSD are apathy, agitation, irritability, aberrant motor activity, anxiety, depression, delusions, hallucinations, euphoria, disinhibition, sleep disturbances, and eating disorders (Chakraborty et al., 2019; Lee, 2020; Savva et al., 2009). BPSD can appear at any stage of the disease, although their presence and intensity increase as the disease progresses to more severe stages (García-Alberca et al., 2010), and they can be concurrent, which makes a significant impact on people with AD and their caregivers (Preuss et al., 2016). Cognitive decline and the presence of BPSD in people with AD are responsible for the deterioration of functional capacity. The affected person gradually loses the ability to perform activities of daily living and becomes dependent on their environment and other caregivers (Desai et al., 2004; Villarejo Galende et al., 2021).
Family caregivers play an important role in the treatment and rehabilitation of people with AD. However, many caregivers routinely experience high worry, stress, and burnout (Bremer et al., 2015; Ruiz-Adame Reina et al., 2017). The presence of BPSD affects caregivers and is associated with a decrease in their quality of life (Whitebird et al., 2013). The daily demands of the person with AD, together with the worries and ruminations related to the course of the disease, lead to caregiver overload that can have negative consequences on mental, physical, social, and financial health (Bremer et al., 2015; Corrêa et al., 2019; Ruiz-Adame Reina et al., 2017). In terms of mental health, the prevalence of clinical depression and anxiety disorders in caregivers of people with AD is 33.9% and 43.6%, respectively, compared to 4.4% and 3.6% in the general population (Ruiz-Adame Reina et al., 2017; World Health Organization, 2017), and is higher than in caregivers of people with other diseases (Atteih et al., 2015; Thunyadee et al., 2015).
As a result, a growing number of interventions are aimed at caregiver care. A recent systematic review and meta-analysis (Cheng et al., 2020) analyzed efficacy in 140 randomized clinical trials (RCTs) of interventions for caregivers of people with dementia.
Nonpharmacological interventions included in the meta-analysis by Cheng et al. (2020) encompassed a wide range of approaches, such as psychoeducation programs, counseling and psychotherapy, mindfulness-based interventions, support groups, care coordination and case management, training of the care recipient with caregiver involvement, multi-component interventions, and miscellaneous interventions. These interventions showed statistically significant improvements in various domains of caregiver well-being, such as reducing overload and stress, alleviating symptoms of depression and anxiety, enhancing subjective well-being, improving caregiver skill and knowledge, promoting positive aspects of caregiving, enhancing physical health, and fostering social support.
Despite these promising results, these programs have several limitations. For example, the effect size estimate for these interventions was small (Hedges’ g between 0.16 and 0.31). Specifically, psychoeducation and symptom management counseling, applied in isolation, have been shown to have transient effects and their implementation in combination with other therapeutic interventions is costly and demanding (Chien & Lee, 2011; Salamin et al., 2019), which reduces treatment participation and adherence rates (Cheng et al., 2020). In this regard, although scientific evidence is still limited, mindfulness-based interventions (MBIs) have been proposed as promising alternative treatment to overcome these limitations (Cheng et al., 2020; Kor et al., 2019).
Mindfulness is defined as the ability to pay mindful attention to present-moment experience with interest, curiosity, and acceptance (Levit-Binnun et al., 2021). Mindfulness practices can play an important role in caregivers’ stress responses and coping skills (Whitebird et al., 2013). MBIs can help caregivers learn to develop an attitude of openness towards their role and enhance their cognitive and emotional abilities. This attitude could favor the processing of highly stressful events, helping to reduce the perceived burden and overall stress (Whitebird et al., 2013).
A recent meta-analysis explored the efficacy of MBIs for caregivers in seven RCTs with a total sample of 258 subjects (Cheng et al., 2020). The results showed that the interventions were effective in alleviating depressive symptoms (Hedges’ g = − 0.58, number of interventions = 7; n = 258) and improving subjective well-being (Hedges’ g = 0.31, number of interventions = 6; n = 212). A more recently published RCT found similar improvements in stress, anxiety, and depression variables in a MBI group versus a control group immediately post-intervention and at 6-month follow-up (Kor et al., 2021).
In these eight RCTs, the caregivers included in the studies were those providing care for individuals clinically diagnosed with dementia, regardless of the specific type of dementia. The interventions implemented in these studies encompassed a variety of MBIs, including Mindfulness-Based Stress Reduction (MBSR) (Brown et al., 2016; Whitebird et al., 2013), modified Mindfulness-Based Cognitive Therapy (Kor et al., 2021), Transcendental Meditation (Leach et al., 2015), Yoga and Meditation Program (Danucalov et al., 2013), and Kirtan Kriya meditation (Lavretsky et al., 2013). Only four studies included follow-up assessments between 3 and 6 months after the intervention. The most commonly assessed variables were quality of life, anxiety, depression, and caregiver overload, and only two studies assessed BPSD in patients. Most of these studies (n = 5) have been carried out in the United States, and the rest in Australia, Brazil, and Hong Kong.
Regarding the indirect effects of MBIs for caregivers on the BPSD of people with dementia, Kor et al. (2021) found significant improvement in BPSD in individuals at post-intervention, evaluated by the Neuropsychiatric Inventory Questionnaire (Cummings, 1997). The study authors concluded that the BPSD would not only be determined by the stage of the disease, but also by the interactions between the person with AD and the caregiver. MBIs could help caregivers not only to improve emotional well-being, but also to respond with greater calm and energy to the person with dementia’s symptoms (Kor et al., 2021).
Considering the characteristics of these previous studies and the differences in the sociodemographic profile of caregivers in Spain (e.g., predominantly female profile), as highlighted by Ruiz-Adame Reina et al. (2017) and Garcia-Ptacek et al. (2019), it seems necessary to continue investigating the efficacy of MBIs in the Spanish population and to consider follow-up measures to explore long-term outcomes. While MBIs often emphasize practices that require sustained attention for extended periods, these exercises can be challenging for AD caregivers and may contribute to feelings of frustration and inadequacy. Unlike other MBIs, the Mindfulness-Based Health Care Program (MBHC) program was specifically designed to address the needs and concerns of caregivers in the context of AD caregiving. This program incorporates practices that are kinder and less demanding in terms of duration, while still focusing on attention and concentration. It places an additional emphasis on cultivating self-compassion and treating oneself with kindness, which is particularly relevant for caregivers who often experience high levels of stress and self-criticism.
Therefore, the present study aimed to examine the effects of an 8-week MBHC program on psychological distress (anxiety and depression symptoms) in family caregivers of people with AD. Furthermore, while previous research has shown the effectiveness of MBIs in alleviating various symptoms in caregivers, few studies have specifically explored the broader impact of MBIs on the complex dynamics between caregivers and care recipients. Thus, this study also aimed to fill a knowledge gap by examining the relationship between BPSD in people with AD and caregiver outcomes. To achieve these objectives, our study outlined the following specific aims: (1) to examine the effects of MBHC on psychological distress (symptoms of anxiety and depression) in caregivers; (2) to evaluate the maintenance of the possible changes in psychological distress in caregivers 3-month post-intervention follow-up; (3) to evaluate the indirect effects of MBHC on BPSD in people with AD; (4) to investigate the maintenance of the possible changes in BPSD in people with AD at 3-month post-intervention follow-up. We proposed a set of hypotheses to examine the expected outcomes of the study. Hypothesis 1: Caregivers who participated in the MBHC program would experience significantly greater reductions in psychological distress (symptoms of depression and anxiety) compared to the TAU control group. Hypothesis 2: These improvements in psychological distress would be maintained and sustained at 3-month follow-up assessment. Hypothesis 3: People with AD whose caregivers had attended MBHC program would exhibit a significant decrease in BPSD. Lastly, Hypothesis 4: The benefits observed in Hypothesis 3, pertaining to the reduction in BPSD, would persist and be maintained at 3-month post-MBHC follow-up assessment.
Method
Participants
Participants were adults aged 18 and older diagnosed with mild cognitive impairment due to AD or dementia due to probable AD according to the National Institute on Aging and Alzheimer’s Association criteria (NIA-AA; McKhann et al., 2011), and their primary current family caregivers. AD was diagnosed by several neurologists at several memory clinics. Participants were recruited from February 2019 until March 2020 (Table 1).
Potential participants read an informed consent document approved by the Institutional Review Board, asked questions, and provided informed consent prior to any data collection and training. For this study, a family caregiver was defined as a non-professional individual who provided informal care in a home setting for a person with AD. This typically included spouses, adult children, and other relatives who played a significant role in supporting and assisting the care recipient in their daily activities. To ensure a more focused analysis of the primary caregiver’s experience, we aimed for a 1:1 ratio of primary caregiver to person with AD.
Inclusion criteria for family caregivers required them to be an informal caregiver, and have a score on the Mini-Mental State Examination (MMSE) higher than 25, which indicates intact normal cognition. People with AD who were on stable medication doses for at least 3 months before their inclusion in the study were also eligible. Both people with AD and their family caregivers were excluded if they reported (1) a neurological disease history (e.g., transient ischemic attack, stroke, meningitis, epilepsy); (2) alcohol and drug abuse, and except tobacco, during the 24 months prior to the start of the study; (3) a systemic disease associated with cognitive impairment (hypothyroidism, B12 deficiency, severe liver or kidney failure, etc.); (4) a severe psychiatric illness (major depression, schizophrenia, etc.); and/or (5) visual and/or auditory perceptual disorders that limit the participants’ ability to complete self-report surveys and the mindfulness-based intervention.
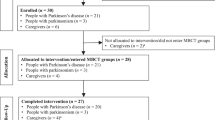
To provide a comprehensive understanding of the participant selection process, we present Fig. 1, which illustrates the selection process of study participants using the CONSORT diagram. This diagram visually represents the various steps involved in participant recruitment and retention. Beginning with the initial selection process, we enrolled a total of 77 participants into the study. Following the allocation of participants into the MBHC and control groups, we conducted assessments at Time 1 to establish baseline measures. These assessments were crucial in capturing the initial characteristics of the caregivers and establishing a starting point for the study. Moving forward to Time 2, which represented the post-intervention and no training stage, we observed that 10 participants from the MBHC group and 6 participants from the control group dropped out. Although the specific reasons for dropouts were not systematically recorded, it is worth noting that participants’ withdrawal may have been influenced by factors such as pre-existing stress levels, individual preferences, and challenges in managing daily life stressors. While some participants in the intervention group reported feeling overwhelmed by the demands of the intervention, others expressed a preference for a different approach. In the control group, dropouts primarily occurred due to participants not responding to attempts for reevaluations, indicating potential difficulties in engaging with the study. Despite these dropouts, we were able to retain a set of 50 (75.6%) caregivers who completed assessments at this stage (Time 2), providing valuable data for analysis. Regrettably, the COVID-19 pandemic posed unforeseen challenges, disrupting the planned follow-up assessments. As a result, 7 caregivers form the MBHC group and 13 caregivers from the control group could not be assessed at the 3-month follow-up. However, we were able to gather data from a final set of 30 (50%) caregivers who completed assessments at Time 3 (3-month follow-up).
Procedure
Potential participants were recruited from the Behavioral Neurology and Dementia Unit of the Hospital San Vicente del Raspeig, the Neurology Service of Hospital Clínico Universitario de San Juan, and the Hospital General Universitario de Alicante; from the primary health centers in Elche, Muchamiel, Santa Pola, and San Juan; and from four local associations of relatives of people with AD in the province of Alicante, Spain. An advertising campaign was also launched by using social networks and posters containing the study information.
Interested caregivers were contacted by telephone and, if they met the eligibility criteria, the person with AD was screened. However, in cases where the person with AD did not meet the eligibility criteria, only the caregiver was included in the study. This allowed us to gather data from caregivers even if the person they cared for was not eligible or chose not to participate. Out of the total number of caregivers contacted, 40 caregivers participated without the person with AD. Following the initial screening, potential participants (both the person with AD and the caregiver) who met the eligibility criteria were scheduled for a face-to-face interview with an occupational therapist. During this meeting, detailed verbal and written information about the research (Online Resource 1) was provided to both the person with AD and their caregiver. If they decided to participate, both parties signed the informed consent form and were assessed for the baseline measurement of the study.
Participants were assigned by simple randomization to the MBHC intervention or the TAU control group (ratio 1:1). Randomization was carried out by generating a random sequence with the randomizeR package of the R statistical software (Uschner et al., 2018). To limit potential selection bias, Eva María Navarrete-Muñoz, who was not involved in the assessments and implementation of the intervention program, was responsible for generating the random sequence. Afterwards, two research assistants informed participants by telephone 1 week before the start of the program which group they had been assigned to. To control for potential bias, the evaluators were unaware of the allocation of the participants in the study, and the MBHC instructor was blinded to the results of the baseline assessments. To safeguard the confidentiality of personal information, each participant received a unique identification number. A research assistant, appointed by the principal investigator, was tasked with overseeing proper data management and storage. Electronic records were regularly backed up and securely stored on a hard drive, while hard copies of original materials, including questionnaires, tests, and personal data, were organized numerically in binders within a secure cabinet. Access to study data is tightly controlled, and all files will be retained in storage for at least 10 years following the conclusion of the study.
Participants completed an assessment at baseline, at 8–10 weeks (post-intervention), and at 20–22 weeks (3-month follow-up). Participants were not paid for their collaboration. To prevent loss to follow-up, caregivers assigned to MBHC were offered a care service if they were accompanied by a family member (e.g., a child or a relative with AD) when attending MBHC sessions. For ethical reasons, after the research was completed, the control group was invited to participate in an online MBHC program free of charge.
The MBHC program shares a similar structure to the Mindfulness-Based Stress Reduction (MBSR) program (Kabat-Zinn, 1990) and incorporates some practices adapted from MBSR. Specifically, the MBSR program includes (1) practices to cultivate attention to the somatic and sensory experience of the present moment and to cultivate a non-reactive and non-judgmental attitude towards the experience, (2) home practice, and (3) once-weekly sessions over 8 weeks with an additional day of silent retreat between Weeks 6 and 7.
Distinctive features of MBHC compared to MBSR include (1) shorter duration of the weekly sessions, lasting 2 hr instead of 2.5–3 hr, and (2) specific practices aimed at cultivating healthy mental habits and healthy prosocial mental habits, including kindness and compassion. A comprehensive description of the MBHC program can be found in the study protocol (Sánchez-Pérez et al., 2022) and on the website https://inteo.umh.es/atenea/.
The MBHC program focuses on (a) paying attention to the present moment; (b) cultivating acceptance and openness to the present experience without resistance and avoidance of judgment; (c) developing and enhancing healthy qualities such as kindness and compassion; and (d) improving deeper self-inquiry by examining subjective experience through thoughts, feelings, and sensations. To provide a nurturing learning environment and facilitate communication with participants, non-violent communication practices (Rosenberg, 2016) and group dynamics based on person-centered facilitation techniques (Rogers, 2018) were used in each program session. All sessions included mindful movement, formal meditation practice, informal meditation practices, sharing personal experiences and thoughts, and explanation of at-home exercises. The content of each of the sessions can be found in Sánchez-Pérez et al. (2022) and as an example, the material of the first session is available on the InTeO research group website: http://inteo.edu.umh.es/atenea/ejemplo-de-sesion-de-mindfulness/.
MBHC groups were formed with between 8 and 15 caregivers, following recommendations from previous studies (Brown et al., 2016; Leach et al., 2015; Oken et al., 2010; Waelde et al., 2017; Whitebird et al., 2013). Participants received printed material at each session and a WhatsApp link to recordings of the meditations to practice at home with the facilitator’s voice. We did not ask participants to record their home practice on paper or through any other means as we aimed to minimize additional demands on their time and avoid adding to their existing stress levels as caregivers. Instead, during the opening session of each upcoming week, participants were encouraged to share their experiences of the week’s practice, fostering a supportive and interactive group environment where participants could reflect, discuss challenges, and learn from each other’s experiences. Considering caregiver overload and motivation to improve adherence to the MBHC program, the 1-day silent retreat was eliminated, following the indications of previous studies with this population (Kor et al., 2019).
To minimize variations in the implementation of the program, all sessions were taught by the same facilitator who designed the program and has 12 years of experience teaching mindfulness programs. To evaluate the implementation process and confirm treatment fidelity, an external observer familiar with the MBHC program used a checklist to assess that the content was taught in each session and recorded the participants’ attendance. However, because of the high levels of commitment and engagement displayed by the participants in the intervention group, it was determined that additional assessments during the session would place a burden on them and impede their uninterrupted participation. As a result, the attendance control was suspended, and the assessment of the content taught in each session was modified. Instead, at the beginning of each session, participants were asked about the previous session and their comments were made thinking aloud, allowing immediate evaluation of their understanding and retention of the content taught.
Participants in the control group continued their treatment as usual (TAU), including the following situations: six-monthly visits to the neurologist, receiving psycho-educational information, visiting the social worker in their area, and so forth.
Measures
All participants completed the study assessments at baseline, post-intervention, and follow-up (3 months). The Spanish version of the Hospital Anxiety and Depression Scale (HADS) (Terol-Cantero et al., 2007) was used to measure psychological distress (i.e., anxiety and depression symptoms) in caregivers. HADS is a self-administered test that assesses anxiety and depression symptoms (Zigmond & Snaith, 1983). The administration of this instrument to different populations has shown that the HADS is a good screening instrument to assess anxiety and depression, and global psychological distress (Terol-Cantero et al., 2015). Caregivers were asked to report their feelings experienced during the last week. HADS consists of 14 items distributed in two independent 7-item subscales: a depression subscale and an anxiety subscale. Each item can be scored on a Likert-type scale ranging from 0 to 3. The total score of each subscale is obtained by adding the individual score of each item, ranging from 0 to 21. The total scale score is calculated from the sum of the two subscales, ranging from 0 to 42. Higher scores are indicative of greater levels of anxiety or depression symptoms, and the total HADS score provides an overall measure of psychological distress. As a screening instrument, the HAD subscales show adequate sensitivity and specificity values (> 76%) in the healthy adult Spanish population at the cut-off point of 10 for the anxiety subscale and 5 for the depression subscale. In this study, Cronbach’s alpha and McDonald’s omega reliability estimates at baseline for total HAD (α = 0.87; ω = 0.92) and for HAD subscales (HAD-depression: α = 0.87; ω = 0.93; HAD-anxiety: α = 0.82; ω = 0.91) were excellent.
The presence of BPSD in people with AD was assessed using the abbreviated Spanish version of the Neuropsychiatric Inventory Questionnaire (NPI-Q) (Boada et al., 2002). This scale allows the identification of clinically significant BPSD and their impact on caregivers’ stress (Kaufer et al., 2000). This questionnaire explores the presence and severity of twelve BPSD: delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor activity, sleep/nighttime behaviors, and appetite and eating habits. Each domain of the NPI-Q includes a screening question indicating the main symptoms of that domain to help the caregiver identify a particular behavior at first. Subsequently, the caregiver is asked to indicate the presence or absence of the BPSD and to rate the severity of the symptoms on a scale of 0 to 3 (0 = not present; 1 = mild; 2 = moderate; and 3 = severe). By adding up all the respective points, the NPI-Q score for presence and severity is obtained which ranges from 0 to 36 points. In the caregiver stress scale, each item is scored from 0 to 5 (0 = no distress; 1 = low distress; 2 = mild distress; 3 = moderate distress; 4 = severe distress; and 5 = extreme distress) and the total score is the sum of the scores for each item, ranging from 0 to 60. In this study, Cronbach’s alpha at baseline was acceptable (NPI-Q severity, α = 0.73; NPI-Q stress, α = 0.72), while McDonald’s omega was good (NPI-Q severity, ω = 0.87; NPI-Q stress, ω = 0.84).
In this study, we collected additional information about the caregivers and the individuals with AD under their care. For the analysis conducted, we included the following sociodemographic features of the caregivers: age, gender, education level, marital status, employment status, living with the family member with AD, months of caregiving, receiving help with household chores, receiving help with care for the relative, and job abandonment. Regarding the individuals with AD, we considered their age, gender, academic level, marital status, and cognitive decline as measured by the Global Deterioration Scale (GDS) (Reisberg et al., 1982). More detailed account of the sociodemographic information, health issues, and aspects related to caregiving among the participants is available at the study protocol (Sánchez-Pérez et al., 2022).
Data Analyses
Data were analyzed using an intention-to-treat and per-protocol approach using the software R, version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). All statistical tests were two-tailed with a significance level set at 0.05.
Missing data were identified at each time point and Little’s missing completely at random (MCAR) test was used to conduct a missing at random analysis for dependent variables. Subsequently, a per-protocol analysis was performed once it was confirmed that missing data occurred randomly, thus ruling out selection bias. Per-protocol analysis included only participants who strictly adhered to the study protocol without any major deviations, providing insights into the efficacy of the intervention under ideal conditions. In contrast, an intention-to-treat analysis (ITT) was performed using two different methods: mean imputation and multiple imputations via Chained Equations. ITT analysis included all participants randomized to treatment groups, regardless of adherence to the study protocol or any subsequent deviations. It aimed to assess the effectiveness of the intervention in real-world scenarios, reflecting the potential outcomes if the intervention were implemented at the population level. This study provides the results of the per-protocol analysis. This approach was chosen to evaluate the intervention’s efficacy under optimal conditions, ensuring a rigorous assessment of treatment outcomes. Additionally, the results of ITT analysis are available on request.
General characteristics of study participants were described as absolute frequency and percentages (categorical variables) and as a mean and standard deviation (SD) when the distribution was normal, or median and interquartile range (IQR) when they were not normally distributed (quantitative variables). The distribution of quantitative variables was assessed using the Lilliefors-corrected Kolmogorov–Smirnov test.
For Hypotheses 1 and 3, the differences between the intervention and control groups regarding symptoms of depression and anxiety in the caregivers and BPSD in people with AD were examined using chi-square test or Fisher’s exact test for categorical variables and the Student t-test or Mann–Whitney U-test for continuous variables. To control confounding bias, linear regression models were used to assess the effect on primary outcomes between study groups using all the significant covariates (p < 0.20) to build the core models. Moreover, following a backward elimination procedure, all the covariates associated with the main outcomes were included at a level of p < 0.10. The previous variables, although not statistically significant, were kept in the models if they changed the magnitude of the main effects by more than 15%. Cohen’s d, when normally distributed, or Cliff’s delta, when not normally distributed, was reported as an index of the size of the effect of MBHC vs TAU on changes in each dependent variable. A larger value of either Cohen’s d or Cliff’s delta indicates a larger effect size. Cohen’s d values around 0.20, 0.50, and 0.80 are commonly interpreted as small, medium, and large effect sizes, respectively (Cohen, 1988). For Cliff’s delta, a value close to 0 suggests no substantial difference between the two groups, while values higher than 0 indicate a strong effect, with values close to 1 indicating complete agreement in ranks and values close to − 1 suggesting a complete reversal of ranks (Cliff, 1993).
For Hypotheses 2 and 4, a mixed model with random effects was estimated to assess the effect of the MBHC intervention on symptoms of depression and anxiety in caregivers and BPSD in people with AD measured at pre-intervention, post-intervention, and follow-up.
Due to the COVID-19 pandemic, the lockdown in Spain started on 14 March 2020, which hindered the implementation of the MBHC program and the assessment of participants. The care centers for people with AD were forced to suspend their non-residential activities. Once they resumed activities for users living in the community, external staff, including caregivers and relatives, were not allowed access to the center. Consequently, the recruitment of new participants and the 3-month follow-up evaluation of the third MBHC group had to be suspended. This decision had significant implications for the statistical power of this study, which originally aimed to recruit 145 participants based on the statistical power analysis (Sánchez-Pérez et al., 2022). However, due to the challenges posed by the pandemic, the study was only able to recruit 66 subjects, falling short of the intended sample size. During the implementation of the MBHC program, three distinct groups were involved. The first two groups diligently completed all three assessments, namely the baseline, post-intervention, and 3-month follow-up evaluations. Regrettably, the disruptions caused by the pandemic hindered the third group from undergoing the 3-month follow-up assessment, limiting their participation to just the baseline and post-intervention assessments. The available data comprises the baseline and post-intervention assessments for a sample size of n = 50 participants. Additionally, for the 3-month follow-up assessment, data is available for a sample size of n = 30 participants.
Results
Preliminary Analyses
Overall, there were no baseline group differences in the sociodemographic and clinical characteristics of the study participants. Regarding caregivers, they ranged in age from 36 to 86 years, with a mean of 59 (SD = 12), were generally women (n = 41, 82%), were married or living with a partner (n = 35, 70%), and, to a greater extent, received help with the care (n = 38, 76%) and continued working (n = 34, 79%). However, it was observed that caregivers allocated in the intervention group have been spending more time (measured in months) caring compared with those in the control group (median = 48 vs. median = 24; p = 0.055). People with AD had a mean age of 80 (SD = 8) and slightly more than half in both groups were women (n = 27, 54%).
The missing data analysis revealed that relative to 66 participants at baseline, post-MBHC, and TAU (Time 2), there were 10 (16.6%) depression screening, anxiety symptoms, and BPSD missing responses.
Differences in Psychological Distress in Caregivers and Behavioral and Psychological Symptoms in People with AD Between the Intervention and Control Groups (Hypotheses 1 and 3)
The results of the MBHC and TAU groups at the three measurement points are summarized in Table 2. Compared to TAU, MBHC resulted in significantly greater decreases in caregiver psychological distress (median = − 3; IQR = − 6.5, 0.0; p = 0.030; Cliff’s δ = − 0.359) and anxiety symptoms (median = − 3; IQR = − 4.5, 1.0; p = 0.043; Cliff’s δ = − 0.033) after the intervention. At the 3-month follow-up, there were no statistically significant differences between the two groups. Compared to TAU, MBHC produced a decrease in the severity of BPSD in people with AD after the intervention, although it was not statistically significant (median = − 2.0; IQR, − 4.5, 0.0; p = 0.069; Cliff’s δ = − 0.30). At 3-month follow-up, there were no statistically significant differences between the two groups.
Effect of MBHC on Psychological Distress in Caregivers and Behavioral and Psychological Symptoms in People with AD (Hypotheses 2 and 4)
The effect of MBHC on psychological distress among caregivers and behavioral and psychological symptoms in people with AD was assessed immediately after the intervention and at 3-month follow-up, as shown in Table 3. Following the intervention, MBHC was associated with a significant reduction in caregivers’ psychological distress (β = − 3.86; 95%CI, − 7.67, − 0.04; p = 0.047). Specifically, we found a reduction in anxiety symptoms (β = − 2.84; 95%CI, − 5.38, − 0.30; p = 0.029), but not in depressive symptoms. However, MBHC did not produce a significant change in psychological distress at 3-month follow-up compared to baseline and post-intervention assessments. Additionally, MBHC did not yield a significant reduction on BPSD in individuals with AD at any point during the study.
Discussion
The goals of this study were to examine the effects of an 8-week mindfulness-based intervention in family caregivers of people with AD and, indirectly, in the patients with AD themselves. To our knowledge, this is the first RCT analyzing the effects of an MBI in this population in Spain.
In relation to the first objective (i.e., to examine the effects of the intervention on psychological distress in caregivers), it was found that caregivers of people with AD showed significantly lower levels of psychological distress at post-intervention, compared to the TAU control group. These results are in line with the study by Sanchez et al. (2020), in which the mindfulness-based intervention showed significant improvement in the HAD total score of caregivers of people with dementia. This result may be related to an improvement in emotional regulation mechanisms (Hervás et al., 2016), specifically in emotional processing, positive reappraisal, reduction of negative repetitive thoughts, improvement in cognitive-emotional reactivity, capacity for acceptance, and self-compassion.
Different authors conclude that the global scale of psychological distress of the HAD works better than the subscales separately (Brennan et al., 2010; Cosco et al., 2012; Herrmann, 1997; Norton et al., 2013; Terol-Cantero et al., 2007, 2015) and that the high correlation between both subscales shows that the items do not effectively differentiate anxiety and depression symptoms (Bjelland et al., 2002). In our study, we observed a significant reduction in anxiety in the MBHC intervention group compared to the control group. This result converges with the studies by Danucalov et al. (2013) and Kor et al. (2021), which reported a significant reduction in anxiety symptoms after MBIs. However, we found no significant changes in depression symptoms in caregivers. This result is consistent with other studies where no significant changes in depression levels were found after the intervention (Danucalov et al., 2013; Kor et al., 2021; Whitebird et al., 2013). Furthermore, several meta-analyses (Burton et al., 2017; Spinelli et al., 2019; van der Riet et al., 2018) agree that the strongest finding of the impact of MBI relates to stress reduction (moderate effect), while for depression the impact is moderate to low.
Still, it should be noted that depressive symptomatology scores on the pre-treatment measure in this study were already relatively low (i.e., initial scores below the cut-off point), and thus, there may have been little room for improvement. Alternatively, the lesser impact of MBHC on depression symptoms might be due to the caregivers in the MBHC group having spent twice as long caring for the person with AD, which may have led to accumulated emotional exhaustion and to a greater number of losses in relation to work activities, social relationships, self-care, leisure, and free time, etc. It should be noted that, although the differences between groups were not statistically significant, the MBHC group started with a higher mean depression score at baseline and had post-intervention depression scores equivalent to the control group, and depression scores that were lower than the control group at the 3-month follow-up.
In relation to the second aim (i.e., to assess whether the changes in psychological distress are maintained at the 3-month follow-up in the intervention group), we observed that the reduction in psychological distress, particularly in anxiety, was not maintained at the 3-month follow-up. While some studies have reported sustained benefits of MBIs on follow-up measures (Goldberg et al., 2022), results in caregiver populations are inconsistent. For instance, a study by Whitebird et al. (2013) found no significant difference in anxiety levels between the intervention and control groups after the intervention and at 4-month follow-up. On the other hand, the study by Kor et al. (2021) observed that the reduction in anxiety and depression levels after an MBI is maintained at 6-month follow-up. Cheng et al. (2020) suggested that while mindfulness programs for caregivers can yield significant and lasting results, sustaining these benefits can be challenging, especially given the severity of stressors faced by caregivers of individuals with AD. Caregivers confront significant daily stressors, which may hinder the long-term maintenance of mindfulness practices due to the demands of caregiving and the progressive nature of AD. Individual factors, such as the integration of mindfulness into daily life and continued engagement in regular practice, may influence the sustainability of mindfulness practices. While ongoing practice was not specifically measured in our study, anecdotal feedback from caregivers suggested that those who integrated mindfulness techniques into their daily routines tended to experience more sustained benefits.
To address these challenges, implementing a maintenance practice group could be beneficial. Such a group would enable participants to continue practicing mindfulness together regularly, fostering social support and accountability. This group dynamic can enhance ongoing practice and contribute to sustaining the effects beyond the program duration.
In relation to the third and fourth objectives (i.e., to assess the effects of MBHC on BPSD in people with AD), we found no statistically significant differences between the MBHC and TAU groups after the intervention and at 3-month follow-up. Only a marginally significant reduction in the severity of BPSD (p = 0.07) with the underpowered sample was found at post-intervention. A study by Kor et al. (2021) assessed BPSD in people with dementia using the NPI-Q and found a statistically significant improvement in both severity of BPSD and caregiver stress caused by BPSD at post-MBI (but not at 6-month follow-up). In contrast, in the study carried out by Oken et al. (2010), no significant effects were observed in these same variables in the post-intervention evaluation. Because too few studies have analyzed the indirect effect of MBI applied to caregivers on the BPSD of patients with dementia, it is too soon to reach a conclusion.
It may seem exaggerated to hypothesize that the improvements generated by an MBI in the emotional well-being of the caregiver can in turn improve the patient’s BPSD, since dementia follows its own evolution and progresses towards more serious stages. However, previous studies have already shown that a closer dyadic relationship between the family caregiver and the person with dementia (the care dyad) can have positive effects and a protective role in the care recipient (Burgener & Twigg, 2002; Fauth et al., 2012; Norton et al., 2009; Perren et al., 2007). In this sense, MBI aimed at caregivers could contribute to improving the care dyad, because if the caregiver responds calmly, acceptingly, and without judgment to the needs of the person with dementia, the quality of the emotional bond between them could improve, and this could support improvement in the BPSD of the care recipient. Studies are needed that examine whether meditation practice in caregivers influences changes in BPSD in care recipients. As noted in Kor et al. (2021), it is important to investigate which MBI-related specific changes in the dyadic caregiver-care recipient interaction are associated with emotional well-being in the caregiver.
Limitations and Future Directions
Due to the COVID-19 pandemic, the final sample size of our study was 50 pairs of caregivers and persons with AD, whereas the original estimated sample size was 145 pairs. Despite this, we did find statistically significant reductions in caregivers’ psychological distress and anxiety after MBHC. Development of online or hybrid MBIs for caregivers (Goodridge et al., 2021; Kor et al., 2022) might help with recruitment of larger samples of caregivers. It will also be important to strengthen and support mindfulness practice once the intervention program is over, to ensure that benefits are maintained. Only regular and continued meditation has a lasting effect on attitudes, moods, and behaviors. Making sure to make meditation a habit by evaluating the factors that make it difficult to practice, looking for the best moments of the day to practice, using smart-phone apps, including informal meditation in daily activities, establishing memory sessions, following up online, and so forth are key for improvements to be maintained over time (Birtwell et al., 2019; Kellen & Saxena, 2020; Laurie & Blandford, 2016; Lea et al., 2014; Li & Leshed, 2022). In addition, it would also be convenient to check, in the follow-up evaluations of the randomized controlled trials, which subjects in the intervention group have continued to practice meditation and which have not, in order to assess whether there are significant differences between the two subgroups with respect to the variables studied.
It is important to note that the sample size in this study was smaller than the ideal size of 145 participants. Of the 66 individuals initially recruited, 50 completed the intervention phase, and unfortunately, the number further decreased to 30 at the 3-month follow-up evaluation. This decrease was primarily because of the impact of the COVID-19 pandemic, which resulted in the suspension of recruitment and affected our ability to reach the desired minimum sample size for adequate statistical power. This reduction in sample size may compromise the study’s statistical power, potentially impacting the ability to detect smaller yet meaningful effects or relationships. While the smaller sample size limits the generalizability of our findings, our study provides valuable preliminary insights into the initial effects of MBHC on participants’ well-being. These findings highlight the potential benefits of MBHC despite the challenges imposed by the smaller sample size. In future studies, larger sample sizes will be essential to enhance statistical power and provide more robust evidence on the effectiveness of MBIs and the specific mechanisms underlying MBHC. By recruiting a larger sample size, we will have the opportunity to conduct a more comprehensive examination of moderators and mediators of MBI-related outcomes. Additionally, we will be able to explore longer-term effects, and assess the content and structure of MBHC in greater detail.
Another limitation of our study was the absence of a silent retreat component in our MBHC program. While including a silent retreat has been reported in some MBIs, we did not find specific studies including a silent retreat in interventions for dementia caregivers. The decision to eliminate the silent retreat was based on careful consideration of the unique circumstances and needs of our AD caregiver participants. Given the demanding nature of caregiving responsibilities and the potential burden that a 1-day retreat could pose, we aimed to optimize their engagement and participation by removing this component. However, it is important to acknowledge that the absence of a silent retreat in our intervention may have differed from other studies and could have potentially influenced our findings. Future research could explore the inclusion of a silent retreat in MBIs for dementia caregivers to examine its potential impact on outcomes.
In this study, we used the HAD scale and the NIP-Q to assess anxiety and depression symptoms in both clinical and community populations (Brennan et al., 2010) including caregivers (Sánchez-López et al., 2015), and BPSDs occurring in people with AD (Cummings, 2020). Future studies might consider using clinical diagnostic interviews to determine clinical diagnoses of anxiety and depression in caregivers.
While our study used self-report tools to measure the effects of MBHC, future studies might benefit from using qualitative interviews to examine in greater depth the perceptions of caregivers of people with AD about mindfulness meditation training and psychological well-being, relationship of the dyadic relationship, on the well-being of the recipient of their care. This methodology has been implemented via focus groups and semi-structured qualitative interviews (Berk et al., 2019; Kor et al., 2022).
We would also acknowledge the absence of formal attendance registers and home practice tracking may represent an important study shortcoming. Initially, we had planned to track attendance and assess the content taught in each session using an external observer and a checklist. The participants in our study were caregivers of individuals with AD, and we recognized the significant demands placed on them in their caregiving roles. We wanted to minimize any additional burden on the caregivers and ensure their uninterrupted engagement and commitment to the intervention. Given the participants’ consistent attendance and active participation in the sessions, we determined that formal attendance registers and recorded home practice data were not necessary for a comprehensive evaluation of treatment fidelity in this context. While the absence of quantitative measures such as attendance registers and home practice tracking may limit the precision of our assessment of participant engagement, we believe that the decision to prioritize the well-being and engagement of the caregivers was justified. Future studies could explore alternative methods that strike a balance between capturing necessary data and minimizing caregiver burden, ensuring a more comprehensive evaluation of participant engagement and treatment fidelity.
Several strengths of our study include the use of a randomized controlled clinical design carried out by a highly qualified multidisciplinary team. In terms of feasibility, it is noteworthy that the groups consisted of 8–15 caregivers, making MBHC a low-cost community-based care program.
The present study extends and contributes to the developing literature on the application of mindfulness with family caregivers of people with AD and its potential indirect effects on BPSD in care recipients. The results show that the MBHC intervention was effective in reducing psychological distress among caregivers, specifically anxiety symptoms. However, these benefits were not maintained at 3-month follow-up assessment. Moreover, no significant improvements were observed in the BPSD of people with AD at the post-intervention or at the 3-month follow-up.
These findings underscore the need for further research. Future studies with larger samples and longer follow-up periods are warranted to verify the long-term effects of MBHC on caregiver well-being and the potential impact on the quality of the dyadic relationship. Additionally, incorporating achievement maintenance interventions, utilizing instruments that specifically assess the dyadic relationship, and employing mixed methodology approaches could further enhance our understanding of the benefits of MBIs in this context.
Furthermore, it is essential to advance our understanding of the underlying mechanisms that explain the efficacy of these programs. By taking this step, it will be possible to develop interventions that have been empirically validated to address the growing issue of caregiver distress within the context of AD. Doing so will enable the development of empirically validated interventions to address the increasingly prevalent problem of caregiver distress in the context of AD.
In conclusion, while this study provides valuable insights into the effectiveness of MBHC in reducing psychological distress among caregivers, it also highlights the need for continued research and the exploration of additional factors that may contribute to improved outcomes. By addressing these gaps, we will be able to provide caregivers with better support and ultimately enhance the well-being of both caregivers and care recipients.
Data Availability
Study data accessibility will be restricted, although data will be available on request. All requests will be reviewed by the principal investigator (A.S.-P.) and the research team and will require a data transfer agreement. Data are not publicly available due to privacy and ethical restrictions.
References
Atteih, S., Mellon, L., Hall, P., Brewer, L., Horgan, F., Williams, D., Hickey, A., ASPIRE-S study group. (2015). Implications of stroke for caregiver outcomes: Findings from the ASPIRE-S study. International Journal of Stroke: Official Journal of the International Stroke Society, 10(6), 918–923. https://doi.org/10.1111/ijs.12535
Berk, L., Warmenhoven, F., Stiekema, A. P. M., van Oorsouw, K., van Os, J., de Vugt, M., & van Boxtel, M. (2019). Mindfulness-based intervention for people with dementia and their partners: Results of a mixed-methods study. Frontiers in Aging Neuroscience, 11, 92. https://doi.org/10.3389/fnagi.2019.00092
Birtwell, K., Williams, K., van Marwijk, H., Armitage, C. J., & Sheffield, D. (2019). An exploration of formal and informal mindfulness practice and associations with wellbeing. Mindfulness, 10(1), 89–99. https://doi.org/10.1007/s12671-018-0951-y
Bjelland, I., Dahl, A. A., Haug, T. T., & Neckelmann, D. (2002). The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research, 52(2), 69–77. https://doi.org/10.1016/s0022-3999(01)00296-3
Boada, M., Cejudo, J. C., Tàrraga, L., López, O. L., & Kaufer, D. (2002). Neuropsychiatric Inventory Questionnaire (NPI-Q): Validación española de una forma abreviada del Neuropsychiatric Inventory (NPI)* [Neuropsychiatric inventory questionnaire (NPI-Q): Spanish validation of an abridged form of the Neuropsychiatric Inventory (NPI)]. Neurologia (barcelona, Spain), 17(6), 317–323.
Bremer, P., Cabrera, E., Leino-Kilpi, H., Lethin, C., Saks, K., Sutcliffe, C., Soto, M., Zwakhalen, S. M. G., Wübker, A., RightTimePlaceCare Consortium. (2015). Informal dementia care: Consequences for caregivers’ health and health care use in 8 European countries. Health Policy (Amsterdam, Netherlands), 119(11), 1459–1471. https://doi.org/10.1016/j.healthpol.2015.09.014
Brennan, C., Worrall-Davies, A., McMillan, D., Gilbody, S., & House, A. (2010). The Hospital Anxiety and Depression Scale: A diagnostic meta-analysis of case-finding ability. Journal of Psychosomatic Research, 69(4), 371–378. https://doi.org/10.1016/j.jpsychores.2010.04.006
Brown, K. W., Coogle, C. L., & Wegelin, J. (2016). A pilot randomized controlled trial of mindfulness-based stress reduction for caregivers of family members with dementia. Aging & Mental Health, 20(11), 1157–1166. https://doi.org/10.1080/13607863.2015.1065790
Burgener, S., & Twigg, P. (2002). Relationships among caregiver factors and quality of life in care recipients with irreversible dementia. Alzheimer Disease and Associated Disorders, 16(2), 88–102. https://doi.org/10.1097/00002093-200204000-00006
Burton, A., Burgess, C., Dean, S., Koutsopoulou, G. Z., & Hugh-Jones, S. (2017). How effective are mindfulness-based interventions for reducing stress among healthcare professionals? A systematic review and meta-analysis. Stress and Health: Journal of the International Society for the Investigation of Stress, 33(1), 3–13. https://doi.org/10.1002/smi.2673
Chakraborty, S., Lennon, J. C., Malkaram, S. A., Zeng, Y., Fisher, D. W., & Dong, H. (2019). Serotonergic system, cognition, and BPSD in Alzheimer’s disease. Neuroscience Letters, 704, 36–44. https://doi.org/10.1016/j.neulet.2019.03.050
Chen, Y., Dang, M., & Zhang, Z. (2021). Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: A systematic review of symptom-general and -specific lesion patterns. Molecular Neurodegeneration, 16(1), 38. https://doi.org/10.1186/s13024-021-00456-1
Cheng, S.-T., Li, K.-K., Losada, A., Zhang, F., Au, A., Thompson, L. W., & Gallagher-Thompson, D. (2020). The effectiveness of nonpharmacological interventions for informal dementia caregivers: An updated systematic review and meta-analysis. Psychology and Aging, 35(1), 55–77. https://doi.org/10.1037/pag0000401
Chien, W. T., & Lee, I. Y. M. (2011). Randomized controlled trial of a dementia care programme for families of home-resided older people with dementia. Journal of Advanced Nursing, 67(4), 774–787. https://doi.org/10.1111/j.1365-2648.2010.05537.x
Cliff, N. (1993). Dominance statistics: Ordinal analyses to answer ordinal questions. Psychological Bulletin, 114(3), 494–509. https://doi.org/10.1037/0033-2909.114.3.494
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Routledge Academic.
Corrêa, M. S., de Lima, D. B., Giacobbo, B. L., Vedovelli, K., de Argimon, I. I. L., & Bromberg, E. (2019). Mental health in familial caregivers of Alzheimer’s disease patients: Are the effects of chronic stress on cognition inevitable? Stress (amsterdam, Netherlands), 22(1), 83–92. https://doi.org/10.1080/10253890.2018.1510485
Cosco, T. D., Doyle, F., Watson, R., Ward, M., & McGee, H. (2012). Mokken scaling analysis of the Hospital Anxiety and Depression Scale in individuals with cardiovascular disease. General Hospital Psychiatry, 34(2), 167–172. https://doi.org/10.1016/j.genhosppsych.2011.11.005
Cummings, J. L. (1997). The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology, 48(5 Suppl 6), S10-16. https://doi.org/10.1212/wnl.48.5_suppl_6.10s
Cummings, J. (2020). The Neuropsychiatric Inventory: Development and applications. Journal of Geriatric Psychiatry and Neurology, 33(2), 73–84. https://doi.org/10.1177/0891988719882102
Danucalov, M. A. D., Kozasa, E. H., Ribas, K. T., Galduróz, J. C. F., Garcia, M. C., Verreschi, I. T. N., Oliveira, K. C., Romani de Oliveira, L., & Leite, J. R. (2013). A yoga and compassion meditation program reduces stress in familial caregivers of Alzheimer’s disease patients. Evidence-Based Complementary and Alternative Medicine: ECAM, 2013, 513149. https://doi.org/10.1155/2013/513149
de Sanchez, M. G. A. P., de Caparrol, A. J. S., de Martins, G., Alves, L. C. S., Monteiro, D. Q., & Gratão, A. C. M. (2020). Mindfulness-based intervention for caregivers of older adults with dementia. SMAD. Revista Eletrônica Saúde Mental Álcool e Drogas, 16(3), 23–32. https://doi.org/10.11606/issn.1806-6976.smad.2020.167674
Desai, A. K., Grossberg, G. T., & Sheth, D. N. (2004). Activities of daily living in patients with dementia: Clinical relevance, methods of assessment and effects of treatment. CNS Drugs, 18(13), 853–875. https://doi.org/10.2165/00023210-200418130-00003
Fauth, E., Hess, K., Piercy, K., Norton, M., Corcoran, C., Rabins, P., Lyketsos, C., & Tschanz, J. (2012). Caregivers’ relationship closeness with the person with dementia predicts both positive and negative outcomes for caregivers’ physical health and psychological well-being. Aging & Mental Health, 16(6), 699–711. https://doi.org/10.1080/13607863.2012.678482
García-Alberca, J. M., Lara Muñoz, J. P., & Berthier Torres, M. (2010). Neuropsychiatric and behavioral symptomatology in Alzheimer disease. Actas Españolas De Psiquiatría, 38(4), 212–222. https://doi.org/10.1016/s0210-4806(10)70045-0
Garcia-Ptacek, S., Dahlrup, B., Edlund, A. K., Wijk, H., & Eriksdotter, M. (2019). The caregiving phenomenon and caregiver participation in dementia. Scandinavian Journal of Caring Sciences, 33(2), 255–265. https://doi.org/10.1111/scs.12627
Gauthier, S., Webster, C., Servaes, S., Morais, J. A., & Rosa-Neto, P. (2022). World Alzheimer Report 2022 – Life after diagnosis: Navigating treatment, care and support. 416.
Goldberg, S. B., Riordan, K. M., Sun, S., & Davidson, R. J. (2022). The empirical status of mindfulness-based interventions: A systematic review of 44 meta-analyses of randomized controlled trials. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 17(1), 108–130. https://doi.org/10.1177/1745691620968771
Goodridge, D., Reis, N., Neiser, J., Haubrich, T., Westberg, B., Erickson-Lumb, L., Storozinski, J., Gonzales, C., Michael, J., Cammer, A., & Osgood, N. (2021). An app-based mindfulness-based self-compassion program to support caregivers of people with dementia: Participatory feasibility study. JMIR Aging, 4(4), e28652. https://doi.org/10.2196/28652
Herrmann, C. (1997). International experiences with the Hospital Anxiety and Depression Scale—A review of validation data and clinical results. Journal of Psychosomatic Research, 42(1), 17–41. https://doi.org/10.1016/s0022-3999(96)00216-4
Hervás, G., Cebolla, A., & Soler, J. (2016). Mindfulness-based psychological interventions and their benefits: Current state of the art. Clínica y Salud, 27(3), 115–124. https://doi.org/10.1016/j.clysa.2016.09.002
Kabat-Zinn, J. (1990). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain and illness. Delacorte.
Kaufer, D. I., Cummings, J. L., Ketchel, P., Smith, V., MacMillan, A., Shelley, T., Lopez, O. L., & DeKosky, S. T. (2000). Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of Neuropsychiatry and Clinical Neurosciences, 12(2), 233–239. https://doi.org/10.1176/jnp.12.2.233
Kellen, M., & Saxena, D. (2020). Calm my headspace: Motivations and barriers for adoption and usage of meditation apps during times of crisis. Proceedings of The 20th International Conference on Electronic Business (pp. 37-46). ICEB’20, Hong Kong SAR, China, December 5- 8.
Kor, P. P. K., Li, M. L., Kwok, D. K. S., Leung, A. Y. M., Lai, D. L. L., & Liu, J. Y. W. (2022). Evaluating the effectiveness of a 6-week hybrid mindfulness-based intervention in reducing the stress among caregivers of patients with dementia during COVID-19 pandemic: Protocol of a randomized controlled trial. BMC Psychology, 10(1), 178. https://doi.org/10.1186/s40359-022-00876-8
Kor, P. P. K., Liu, J.Y.-W., & Chien, W. T. (2019). Effects on stress reduction of a modified mindfulness-based cognitive therapy for family caregivers of those with dementia: Study protocol for a randomized controlled trial. Trials, 20(1), 303. https://doi.org/10.1186/s13063-019-3432-2
Kor, P. P. K., Liu, J. Y. W., & Chien, W. T. (2021). Effects of a modified mindfulness-based cognitive therapy for family caregivers of people with dementia: A randomized clinical trial. The Gerontologist, 61(6), 977–990. https://doi.org/10.1093/geront/gnaa125
Laurie, J., & Blandford, A. (2016). Making time for mindfulness. International Journal of Medical Informatics, 96, 38–50. https://doi.org/10.1016/j.ijmedinf.2016.02.010
Lavretsky, H., Epel, E. S., Siddarth, P., Nazarian, N., Cyr, N. S., Khalsa, D. S., Lin, J., Blackburn, E., & Irwin, M. R. (2013). A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. International Journal of Geriatric Psychiatry, 28(1), 57–65. https://doi.org/10.1002/gps.3790
Lea, J., Cadman, L., & Philo, C. (2014). Changing the habits of a lifetime? Mindfulness meditation and habitual geographies. Cultural Geographies, 22, 49–65. https://doi.org/10.1177/1474474014536519
Leach, M. J., Francis, A., & Ziaian, T. (2015). Transcendental meditation for the improvement of health and wellbeing in community-dwelling dementia caregivers [TRANSCENDENT]: A randomised wait-list controlled trial. BMC Complementary and Alternative Medicine, 15, 145. https://doi.org/10.1186/s12906-015-0666-8
Lee, G. (2020). Impaired cognitive function is associated with motor function and activities of daily living in mild to moderate Alzheimer’s dementia. Current Alzheimer Research, 17(7), 680–686. https://doi.org/10.2174/1567205017666200818193916
Levit-Binnun, N., Arbel, K., & Dorjee, D. (2021). The mindfulness map: A practical classification framework of mindfulness practices, associated intentions, and experiential understandings. Frontiers in Psychology, 12, 727857. https://doi.org/10.3389/fpsyg.2021.727857
Li, J., & Leshed, G. (2022). Beyond meditation: Everyday mindfulness and technology use. Extended Abstracts of the 2022 CHI Conference on Human Factors in Computing Systems, 1–6. https://doi.org/10.1145/3491101.3519820
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Kawas, C. H., Klunk, W. E., Koroshetz, W. J., Manly, J. J., Mayeux, R., Mohs, R. C., Morris, J. C., Rossor, M. N., Scheltens, P., Carrillo, M. C., Thies, B., Weintraub, S., & Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7(3), 263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Niu, H., Álvarez-Álvarez, I., Guillén-Grima, F., & Aguinaga-Ontoso, I. (2017). Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurología (barcelona, Spain), 32(8), 523–532. https://doi.org/10.1016/j.nrl.2016.02.016
Norton, M. C., Piercy, K. W., Rabins, P. V., Green, R. C., Breitner, J. C. S., Ostbye, T., Corcoran, C., Welsh-Bohmer, K. A., Lyketsos, C. G., & Tschanz, J. T. (2009). Caregiver-recipient closeness and symptom progression in Alzheimer disease. The Cache County Dementia Progression Study. The Journals of Gerontologz Series B, Psychological Sciences and Social Sciences, 64(5), 560–568. https://doi.org/10.1093/geronb/gbp052
Norton, S., Cosco, T., Doyle, F., Done, J., & Sacker, A. (2013). The Hospital Anxiety and Depression Scale: A meta confirmatory factor analysis. Journal of Psychosomatic Research, 74(1), 74–81. https://doi.org/10.1016/j.jpsychores.2012.10.010
Oken, B. S., Fonareva, I., Haas, M., Wahbeh, H., Lane, J. B., Zajdel, D., & Amen, A. (2010). Pilot controlled trial of mindfulness meditation and education for dementia caregivers. Journal of Alternative and Complementary Medicine (New York, N.Y.), 16(10), 1031–1038. https://doi.org/10.1089/acm.2009.0733
Olazaran-Rodriguez, J., Aguera-Ortiz, L. F., & Muniz-Schwochert, R. (2012). Sintomas psicologicos y conductuales de la demencia: Prevencion, diagnostico y tratamiento [Psychological and behavioural symptoms of dementia: Prevention, diagnosis and treatment]. Revista De Neurologia, 55(10), 598–608.
Perren, S., Schmid, R., Herrmann, S., & Wettstein, A. (2007). The impact of attachment on dementia-related problem behavior and spousal caregivers’ well-being. Attachment & Human Development, 9(2), 163–178. https://doi.org/10.1080/14616730701349630
Preuss, U. W., Wong, J. W. M., & Koller, G. (2016). Treatment of behavioral and psychological symptoms of dementia: A systematic review. Psychiatria Polska, 50(4), 679–715. https://doi.org/10.12740/PP/64477
Reisberg, B., Ferris, S. H., de Leon, M. J., & Crook, T. (1982). The Global Deterioration Scale for assessment of primary degenerative dementia. The American Journal of Psychiatry, 139(9), 1136–1139. https://doi.org/10.1176/ajp.139.9.1136
Rogers, C. R. (2018). Grupos de encuentro. Amorrortu editores.
Rosenberg, M. (2016). Comunicación no violenta: Un lenguaje de vida. Editorial Acanto S.A.
Ruiz-Adame Reina, M., González-Camacho, M. C., Romero-García, J. E., & Sánchez-Reyes Fernández, L. M. (2017). Profiles of Alzheimer’s caregivers in Spain: Social, educational and laboral characteristics. Scandinavian Journal of Caring Sciences, 31(4), 867–877. https://doi.org/10.1111/scs.12408
Salamin, V., Ray, P., Gothuey, I., Corzani, S., & Martin-Soelch, C. (2019). An internet-based intervention for the relatives of people with mental illnesses: An open pilot trial with two groups. Swiss Journal of Psychology, 78(1–2), 15–27. https://doi.org/10.1024/1421-0185/a000219
Sánchez-López, M. P., Limiñana-Gras, R. M., Colodro-Conde, L., & Cuéllar-Flores, I. (2015). Use of the Hospital Anxiety and Depression Scale in Spanish caregivers. Scandinavian Journal of Caring Sciences, 29(4), 751–759. https://doi.org/10.1111/scs.12206
Sánchez-Pérez, A., Mendialdua-Canales, D., Hurtado-Pomares, M., Peral-Gómez, P., Juárez-Leal, I., Espinosa-Sempere, C., Fernández-Pires, P., Zango-Martín, I., Abellán-Miralles, I., López-González, P., Valera-Gran, D., & Navarrete-Muñoz, E.-M. (2022). The ATENción Plena en Enfermedad de Alzheimer (ATENEA-Mindfulness in Alzheimer’s Disease) program for caregivers: Study protocol for a randomized controlled trial. Healthcare (basel, Switzerland), 10(3), 542. https://doi.org/10.3390/healthcare10030542
Savva, G. M., Zaccai, J., Matthews, F. E., Davidson, J. E., McKeith, I., Brayne, C., Medical Research Council Cognitive Function and Ageing Study. (2009). Prevalence, correlates and course of behavioural and psychological symptoms of dementia in the population. The British Journal of Psychiatry: The Journal of Mental Science, 194(3), 212–219. https://doi.org/10.1192/bjp.bp.108.049619
Spinelli, C., Wisener, M., & Khoury, B. (2019). Mindfulness training for healthcare professionals and trainees: A meta-analysis of randomized controlled trials. Journal of Psychosomatic Research, 120, 29–38. https://doi.org/10.1016/j.jpsychores.2019.03.003
Terol-Cantero, M. C., Cabrera-Perona, V., & Martín-Aragón, M. (2015). Revisión de estudios de la Escala de Ansiedad y Depresión Hospitalaria (HAD) en muestras españolas. Anales De Psicología, 31(2), 494–503. https://doi.org/10.6018/analesps.31.2.172701
Terol-Cantero, M. C., López-Roig, S., Rodríguez-Marín, J., Gelabert, M., Pastor, M. -Á., & Reig, M. T. (2007). Propiedades psicométricas de la Escala Hospitalaria de Ansiedad y Estrés (HAD) en población española. Ansiedad y Estrés, 13, 163–176.
Thunyadee, C., Sitthimongkol, Y., Sangon, S., Chai-Aroon, T., & Hegadoren, K. M. (2015). Predictors of depressive symptoms and physical health in caregivers of individuals with schizophrenia. Nursing & Health Sciences, 17(4), 412–419. https://doi.org/10.1111/nhs.12205
Uschner, D., Schindler, D., Hilgers, R.-D., & Heussen, N. (2018). RandomizeR: An R package for the assessment and implementation of randomization in clinical trials. Journal of Statistical Software, 85(8), 1–22. https://doi.org/10.18637/jss.v085.i08
van der Riet, P., Levett-Jones, T., & Aquino-Russell, C. (2018). The effectiveness of mindfulness meditation for nurses and nursing students: An integrated literature review. Nurse Education Today, 65, 201–211. https://doi.org/10.1016/j.nedt.2018.03.018
Villarejo Galende, A., Eimil Ortiz, M., Llamas Velasco, S., Llanero Luque, M., de Silanes, L., de Miguel, C., & Prieto Jurczynska, C. (2021). Report by the Spanish Foundation of the Brain on the social impact of Alzheimer disease and other types of dementia. Neurología, 36(1), 39–49. https://doi.org/10.1016/j.nrl.2017.10.005
Waelde, L. C., Meyer, H., Thompson, J. M., Thompson, L., & Gallagher-Thompson, D. (2017). Randomized controlled trial of inner resources meditation for family dementia caregivers. Journal of Clinical Psychology, 73(12), 1629–1641. https://doi.org/10.1002/jclp.22470
Whitebird, R. R., Kreitzer, M., Crain, A. L., Lewis, B. A., Hanson, L. R., & Enstad, C. J. (2013). Mindfulness-based stress reduction for family caregivers: A randomized controlled trial. The Gerontologist, 53(4), 676–686. https://doi.org/10.1093/geront/gns126
World Health Organization. (2017). Depression and other common mental disorders: Global health estimates. Retrieved September 8, 2022, from https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf
World Health Organization. (2021). Global status report on the public health response to dementia. World Health Organization. Retrieved September 8, 2022, from https://apps.who.int/iris/bitstream/handle/10665/344701/9789240033245-eng.pdf
Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
Acknowledgements
We are truly grateful to all the entities involved in recruiting the sample for this study: the Behavioral Neurology and Dementia Unit of the San Vicente Hospital, General University Hospital of Alicante, and University Clinic Hospital of San Juan; the primary health centers in Santa Pola, San Juan, and Muchamiel; and from four local Associations of relatives of Alzheimer’s patients (Asociación de Familiares y amigos de enfermos de Alzheimer, AFA) in the province of Alicante: AFA Alicante, AFA Elche, AFA Santa Pola, and AFA Campello.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was partially supported by the Vice-Rector for Research of Miguel Hernández University (Ayudas de difusion de la Ciencia).
Author information
Authors and Affiliations
Contributions
Alicia Sánchez-Pérez: preregistration; conceptualization; methodology; investigation; data curation; formal analysis; writing—original draft preparation; writing—review and editing; visualization; supervision; project administration. María Paz Quesada-Rico: investigation; writing—review and editing. Daniel Mendialdua-Canales: methodology. Miriam Hurtado-Pomares: writing—review and editing. Eva María Navarrete-Muñoz: conceptualization; methodology; formal analysis; writing—review and editing; supervision. Desirée Valera-Gran: conceptualization; methodology; writing—original draft preparation; writing—review and editing. Paula Peral-Gómez: writing—review and editing. Gemma Benavides Gil: writing—review and editing. Pablo García Millán: data curation; formal analysis. Gloria González-Caballero: investigation; writing—review and editing. Covadonga Chaves-Vélez: writing—review and editing. Philippe Goldin: supervision; writing—review and editing.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the Research Ethics Committees of the Universidad Miguel Hernández (registration 2017.413.E.OEP; 2017.470.E.OEP), Hospital Universitario San Juan de Alicante (code 18/317), and Hospital General Universitario de Elche (code 44/2019). The research was conducted according to the guidelines of the Declaration of Helsinki.
Consent to Participate
All participants received verbal and written information about the research (Appendix 1) and provided written informed consent.
Conflict of Interest
The authors declare no competing interests.
Additional information
Use of Artificial Intelligence
Artificial intelligence (AI) was not used.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sánchez-Pérez, A., Quesada-Rico, M.P., Mendialdua-Canales, D. et al. Effect of an 8-Week Mindfulness Meditation Training Program on Psychological Distress in Caregivers and on Behavioral and Psychological Symptoms in People with Alzheimer’s Disease: A Randomized Controlled Trial. Mindfulness 15, 1289–1304 (2024). https://doi.org/10.1007/s12671-024-02374-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12671-024-02374-x