Abstract
Objectives
Previous meta-analyses suggested that mindfulness-based interventions (MBIs) may have beneficial effects on telomere length (TL) and telomerase activity (TA), two biological markers of cellular aging and cell stress. The present review aimed to provide the most comprehensive synthesis of the available evidence to date and tested a number of important effect moderators.
Method
Twenty-five studies (18 RCTs, 1 RCT and cohort study, 6 non-randomized studies) with 2099 participants in total were obtained with a systematic literature search, 10 studies had not been included in any previous meta-analysis. Effect sizes were aggregated with random-effects models, the risk of bias was evaluated with standardized checklists, and the most influential moderators were identified with a machine-learning approach.
Results
On average, MBIs had small-to-medium effects on TL (g = 0.23, 95% CI = [0.07, 0.39], p = 0.006) and TA (g = 0.37 [0.01, 0.73], p = 0.046), which, however, were driven by retrospective case–control studies with experienced meditators (TL) and by studies without control interventions and studies from Asia (TA). Most studies had an unclear risk of bias and low analytic power, and there was an indication of publication bias among the TL studies.
Conclusions
TL may not be a useful outcome to assess the efficacy of common MBIs. Effects on TA were smaller than previously assumed and may not be specific for MBIs; TA likely is increased by other active interventions as well. More high-quality and high-powered studies, which also apply open-science practices, are needed to move the field forward.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mindfulness has its roots in Buddhist teachings with the goal of achieving “a state of transcendent bliss and peace” (Bodhi, 2011, p. 21). In current Western research and practice the term mindfulness acts more as an umbrella term, encompassing different practices, processes, and characteristics that are all connected to the concepts of attention, awareness, memory/retention, and acceptance/discernment (Van Dam et al., 2018). In recent years, mindfulness as well as associated practices, like meditation techniques and yoga, have become popular tools for stress reduction and improving mental health (Goldberg et al., 2022; Van Dam et al., 2018). Mindfulness-based interventions (MBIs), such as Mindfulness-Based Stress Reduction (Kabat-Zinn, 1990), have also been employed in clinical settings to improve symptoms of anxiety, depression, and other health issues (Goldberg et al., 2022). The mechanisms through which MBIs influence mental health likely lie in the combination of enhancing positive emotional regulation strategies and self-compassion as well as decreasing rumination and experiential avoidance (Chiesa et al., 2014). This, in turn, may lead to a reduction of stress and better health. Other models of change highlight, for example, the four interrelated components of attention regulation, body awareness, emotion regulation, and change in the perspective on the self (Hölzel et al., 2011); self-awareness, self-regulation, self-transcendence, and six underlying neurocognitive networks (S-ART; Vago & Silbersweig, 2012); or attention monitoring and acceptance (MAT; Lindsay & Creswell, 2017) to explain the effects of MBIs on mental health (for an overview on these models, see also Tran et al., 2022).
It has been hypothesized that mindfulness meditation could also affect cellular aging. Epel et al. (2009) proposed a model according to which mindfulness meditation affects telomere length (TL) through stress reduction by changing cognitive appraisal and decreasing rumination. Telomeres are repetitive nucleotide sequences that protect chromosomal ends from deterioration and preserve DNA material (Chan & Blackburn, 2004). TL typically decreases with age and is affected by life experiences as well as psychological and behavioral factors (Lin et al., 2012; Putterman & Epel, 2012). A reduction in TL is associated with higher stress levels (Bojesen, 2013) and shorter telomeres predict mortality and aging-related diseases (Blackburn et al., 2015) as well as psychological disorders (Epel & Prather, 2018). Previous meta-analyses (Dunn & Dimolareva, 2022; Schutte et al., 2020; Table 1) indeed provide support for a small but consistent effect of MBIs on telomere length.
TL can be restored through the enzyme telomerase (Chan & Blackburn, 2004), which is an important protective factor against biological aging. Like TL, telomerase activity (TA) is affected by stress, but it is still unclear whether stress leads to lower or higher activity, as both high and low levels of TA have been observed under stress exposure (Epel, 2012). Yet, increases of TA in response to stress may counteract telomere shortening and could therefore serve as an indicator of cellular stress (Epel, 2012).
Two meta-analyses (Dunn & Dimolareva, 2022; Schutte & Malouff, 2014; Table 1) have confirmed beneficial medium-to-large-sized effects of MBIs concerning increased TA. The interplay between TL, TA, stress, and declining health (Epel & Prather, 2018) could thus provide a biological explanation for the beneficial health effects of MBIs.
There are currently in total three extant meta-analyses and two systematic reviews on the effects of MBIs on TL and TA (Table 1). Previous reviews differed in investigated outcomes, the design of included primary studies, and number of included studies. Additionally, there was a large outlier in Schutte et al. (2020), which possibly hints at a reporting or coding error. Information about the risk of bias in individual studies was missing in Schutte et al. (2020) and Schutte and Malouff (2014). The systematic review by Dasanayaka et al. (2021) focused only on healthy subjects, while the systematic review of Black and Slavich (2016) focused more on immune-related biomarkers and only included few studies assessing telomere length or telomerase activity. Both did not provide any statistical synthesis. Only primary studies written in English were eligible for inclusion in all of the four prior reviews. This may have resulted in language bias and the exclusion of further relevant studies.
In conclusion, each of the previous reviews addressed only part of the available evidence and could not provide detailed or high-powered analyses on effect moderators, because of the relatively small numbers of included studies. Potential moderators include study characteristics (e.g., publication year, study design, sample size, number of dropouts, study quality, conflicts of interest), participant characteristics (e.g., age, sex, health status), aspects of the intervention (e.g., type and amount of meditation, if alone or in a group, at home or elsewhere), and the control condition. Participant, intervention, and control characteristics (cf. PICO: participants, intervention, comparison, outcome; O’Connor et al., 2008) may all be relevant for the apparent magnitude of reported effects (Goldberg et al., 2022). Furthermore, study characteristics may act as effect moderators, because they are related to the risk of bias and statistical power (e.g., study design, sample size, number of dropouts, study quality, conflicts of interest) and possible decline effects (publication year; see Protzko & Schooler, 2017).
Hence, the present review aimed to provide (1) a comprehensive synthesis of all available evidence of the effects of MBIs on TL and TA and (2) more detailed and higher-powered moderator analyses. We investigated the following three research questions (RQs): What are the overall effects of MBIs on TL (RQ1) and TA (RQ2)? Which study and sample characteristics may explain heterogeneity in the results of primary studies (RQ3)? We included both randomized controlled trials (RCTs) and quasi-experimental studies, comparing either an MBI with a control condition or experienced meditators with non-meditators, and all types of samples (healthy, psychiatric, or other medical) to test the generality of effects.
Method
We adhered to PRISMA (Moher et al., 2009) in the description of the methods used in this meta-analysis. The full analysis code is provided in supplementary materials. The PRISMA-P protocol (Shamseer et al., 2015) of the present study can be found on the OSF repository (https://osf.io/827uk/).
Search Strategy and Study Selection
To identify relevant studies, we used five electronic databases (PsychINFO, Pubmed, Clinical Key, CINHAL Complete, and Google Scholar) as well as the reference lists of the three previously published reviews (Dasanayaka et al., 2021; Dunn & Dimolareva, 2022; Schutte & Malouff, 2014; Schutte et al., 2020). For the electronic database search, we employed a multi-tiered search strategy. The first stage included only the keywords “mindful*,” “meditation,” and “telomere*” (as we initially intended to assess only TL). For this search, PsychINFO, Pubmed, Clinical Key, CINHAL Complete, and Google Scholar (screening the first 1,000 results for eligibility) were used. Searches were conducted on November 8, 2021, with Google Scholar and on November 9, 2021, with the other databases. Only studies in English were included in this first stage. The second stage of the literature search utilized only Google Scholar, because the other databases had not provided any further studies that were not also recovered with Google Scholar in the first stage. In this second search stage (conducted on March 4, 2022, instead of April 3, 2022, as erroneously stated in the preregistration), we used the terms “mindful*,” “meditation,” “telomere*,” and “telomerase*” without any language restrictions. The first 500 studies were screened for eligibility. Both stages of the literature search were conducted independently and in duplicate by two reviewers. The interrater agreement of study selection was 97.6%. The reviewers discussed disagreements until they reached a consensus.
Inclusion Criteria
Studies were selected according to the following inclusion criteria: studies (1) used an RCT or a retrospective or prospective quasi-experimental design, (2) deployed an MBI or similar form of mind–body intervention, and (3) reported outcomes for TL and/or TA with (4) enough statistical information to compute an effect size. We excluded studies with A versus A + B designs (e.g., meditation vs. meditation + medication; to ensure that interventions were not too similar) or if they did not include a control condition (i.e., single-arm studies). There were no exclusion criteria regarding participant characteristics, settings, language, publication status, or publication years. Also, no limitations about the time span between baseline and post-interventional measurements in studies were set.
Quality Assessment
Risk of bias assessments was adopted from prior reviews (Dasanayaka et al., 2021; Dunn & Dimolareva, 2022) for studies, which had been included in these reviews. Independent assessments were conducted for studies, which had not been rated before. Risk of bias was assessed using the Cochrane risk of bias tool (Higgins & Altmann, 2008) for RCTs, and the Joanna Briggs Institute checklists for case–control studies, cohort studies, and quasi-experimental studies (Moola et al., 2020; Munn et al., 2020). Since blinding of participants was not possible in the present suite of studies, we excluded this category from the quality ratings. Judgments were made independently and in duplicate by two reviewers and disagreements were resolved through discussion (also with the other authors). Interrater agreement for the risk of bias assessment was 89.9%. We used the overall risk judgment for each study for statistical analysis.
Data Extraction
The coding scheme was developed before data extraction from the studies selected in the first search stage. It was modified before data extraction from the studies of the second search stage to include information about TA. We extracted the following data from each study. Coded study characteristics were: publication year, publication status, country, setting, study design, type of effect size (see Summary Measures), n of total study and study arm, number and percent of dropouts, study quality (see next section), and conflicts of interest (as reported in the studies themselves). Participant characteristics included: mean age, percent women, and health status (whether participants had diagnosed illnesses); coded aspects of the interventions were: type and style of the mindfulness intervention, dosage, intervention elements concerning whether participants mediated on site and/or at home or were experienced meditators, meditation guidance (e.g., in person or via video), the delivery of the intervention (in group or alone), and the control condition. For the calculation of effect sizes, we further extracted information on the type of outcome measure (TA/TL) and the required statistics (post-interventional means, change score means, SDs) of the intervention and control groups.
Two reviewers extracted the data independently and in duplicate from each eligible study. Interrater agreement of the coding was 93.0%. Disagreements were resolved upon discussion. The corresponding author of one study (Puhlmann et al., 2019) was contacted via email for missing information, but we received no reply.
Data Analyses
For TL and TA, outcomes were analyzed using Hedges’ g (with 95% CI), calculated for the post-interventional differences between intervention and control groups (Borenstein et al., 2021, p. 26–27, Formulas 4.18 and 4.22):
Where df = n1 + n2 and n1, n2, M1, and M2 are the sample sizes and post-interventional means of the two groups and SDpooled their pooled within-subject standard deviation. Intervention groups were assigned to Group 1, whereas control groups to Group 2. For studies reporting only change scores, an effect size measure for pretest–posttest-control group designs was calculated (Morris, 2008, Formula 5):
Where n1, n2, M1, M2, SD1, and SD2 are the sample sizes, mean change scores, and standard deviations of the change scores in the two groups. For the calculation of the variance of this effect size, Formula 16 from Morris (2008) was used, setting the unreported correlation between pretest and posttest scores to r = 0.5 (as in other meta-analyses in the field, e.g., Goldberg et al., 2019; using other values also did not meaningfully impact results).
For the calculation of the overall effect size and its variance from Duraimani et al. (2015), who reported data from two independent markers, Formulas (29.1) and (29.2) from Borenstein et al., (2021, p. 265) were used to arrive at a single effect size, setting for the calculation of the variance r = 0.5 for the unreported correlation between the effect sizes. For the calculation of the overall effect size and its variance from Mason et al. (2018), who reported data from five independent markers, Formulas (29.4) and (29.6) from Borenstein et al., (2021, p. 268) were used (setting again r = 0.5).
Data analysis was conducted with the R packages metafor (Viechtbauer, 2010) and metaviz (Kossmeier et al., 2020a) for visualization. Significance was set to p < 0.05. Numbers are presented with two decimal places, except where needed with more places to provide sufficient accuracy; for p-values < 0.05, which are displayed with three decimal places; and for mean sample age, which is displayed with one decimal place. Multivariate random-effects models were used for the meta-analytic aggregation of the effect sizes, since there was non-independent data (Carlson et al., 2015, Epel et al., 2016, and Le Nguyen et al., 2019, reported comparisons of more than two groups each which introduced non-independence of effect sizes within these studies) and studies varied in important characteristics, like intervention type or dosage. In contrast to the fixed-effect model, effect sizes in the random-effects model are not expected to have the same true effect size. Instead, studies’ true effects are modeled as random, which implies that besides the meta-analytic summary effect also the variance of the studies’ true effect sizes is estimated from the data. Thus, the observed effect size variance is partitioned into between-study variance (true effect size heterogeneity) and within-study variance (sampling error) in the random-effects model (Borenstein et al., 2021). The between-study variance affects the weights for the computation of the meta-analytic summary effect and its variance. For the construction of the required approximate variance–covariance matrix for this multivariate model, we followed instructions by Viechtbauer (n.d.-a, n.d.-b).
Heterogeneity was assessed with the Q test, the I2 statistic, and 95% prediction intervals. The Q test tests the null hypothesis that all studies share a common effect size (Borenstein et al., 2021). The I2 statistic quantifies the percentage of the total variance that reflects true effect size heterogeneity. It gives an overview of how much of the observed variance would remain if sampling errors were eliminated (Borenstein et al., 2017). The 95% prediction interval reflects the interval into which the effect of a new study would fall in 95% of all cases if the study was selected from a random sample (Borenstein et al., 2017). Evidence for heterogeneity was based on the following criteria: A significant Q test and/or I2 > 25% and/or a prediction interval containing zero (as this would imply the possibility of directionally opposing effects in new studies).
For gauging the risk of bias across studies, we present sunset funnel plots (Kossmeier et al., 2020b), which graphically display the individual studies’ power in detecting the summary effect and provide tests of excess significance (TES; Ioannidis & Trikalinos, 2007) and an index of the expected replicability of results (R-index; Schimmack, 2016). However, the TES was computed per hand for TA, since studies were significant in both directions (negative and positive), but only positive results were relevant in the present case, which, however, could not be specified in the software. The use of sunset funnel plots diverged from the preregistered analysis plans but was decided on for ease of applicability and interpretability. We originally planned using recommendations provided in the shiny app by Carter et al. (2019) in helping us select the methods to investigate publication bias. Additionally, we performed robustness and sensitivity checks for outliers (using methods implemented for outlier detection in metafor).
Moderator analyses were conducted via meta-regression analyses with the following variables for both TL and TA: (1) publication year; (2) publication status (published/unpublished); (3) region (North America & Australia/Europe/Asia); (4) setting (clinical/non-clinical); (5) study design (RCT/quasi-experimental); (6) type of effect size (differences in gain scores/post-interventional differences); (7) study n; (8) %dropouts; (9) study quality (low risk/unclear risk/high risk); (10) conflicts of interest (not reported/reported); (11) participant mean age; (12) %women; (13) participant health (illness/no illness); (14) meditation style (modern/traditional); (15) dosage (hours of meditation); (16) intervention elements (experienced meditators/on site & at home/on site & optionally at home/on site); (17) meditation guidance (in person/written, audio, or video); (18) intervention delivery (group/alone/alone & in group); (19) control condition (no intervention/active intervention or experienced meditators). Since the study design included only one non-randomized prospective study (cohort study) for TL and TA each, we combined it with non-randomized retrospective studies (case–control studies) and contrasted it with all randomized prospective studies (RCTs). Experienced meditators as control group were combined with active intervention controls, as we assumed that meditators did not pause in their meditation practice for the duration of the study (at least, this was not indicated in the included primary studies).
Additionally, we utilized a machine-learning approach to assess the relative importance of the moderators in explaining effect-size heterogeneity. We therefore conducted a random forest analysis with the R package metaforest (van Lissa, 2020a), utilizing default settings (100-fold replicated feature selection, retaining only moderators with positive variable importance in > 10% of replications; 5000 regression trees with random-effect weights). Variables with missing data in some studies were excluded from this analysis. For TL, we excluded conflict of interest, %dropout, %women, meditation guidance, and intervention delivery and for TA conflict of interest, meditation guidance, and intervention delivery. This analysis is capable of identifying the most important predictors even under conditions of a low studies-to-moderators ratio.
Results
Study Selection
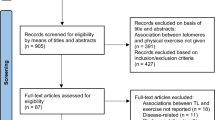
The literature search led to the inclusion of 25 studies in total, of which 10 were not used in previous reviews. A PRISMA flowchart with detailed information is provided in Figure S1 in Supplementary Materials. During the coding process, we became aware of and included an additional study (Dasanayaka et al., 2022) that we did not find otherwise in the literature search. The two main reasons for study exclusions were: Studies did not provide sufficient statistical information to calculate an effect size (k = 13) or did not report any assessment of TL and/or TA (k = 9).
Study Characteristics
The 25 studies included 2,099 participants in total. All studies were published in English, 18 studies reported RCTs, one study both an RCT and a cohort study, and most studies (k = 16) were conducted in non-clinical settings. The duration of the intervention (or meditation experience of participants) varied between four days and 925 weeks with a median of 10 weeks across studies. All studies devised some sort of mindfulness-based intervention, ranging from MBSR (Kabat-Zinn, 1990) to acceptance and commitment therapy (ACT; Hayes et al., 1999) in the case of modern Western interventions, and various meditation trainings in the case of traditional interventions. Five studies investigated experienced meditators with case–control designs. Further three studies investigated the effects of retreats with experienced meditators, two of which used experienced meditators also as a control group. There was therefore substantial variation in hours of meditation between studies, the lowest being six hours and the highest at 6477 h. Controls did not receive any treatment (k = 14), were experienced meditators themselves (k = 2), or received interventions that were unrelated to meditation (k = 9), such as cognitive behavioral therapy (Wang et al., 2017) or health education (Ho et al., 2012). Most studies (k = 12) assessed TL as their outcome of interest, seven studies TA, and another six studies assessed both TL and TA. Hendrich (2019) reported data from only 25 participants even though 39 participants finished the follow-up. We interpreted this difference as a dropout in the current study. For further study information, see Table 2. Results of individual studies are provided in separate forest plots for TL and TA (Supplementary Material, Figures S2 and S3).
Detailed information about the risk of bias within studies can be found in Supplementary Materials (Figures S4 to S8). Among the RCTs (Figures S4 and S5), 14 out of 19 studies had an unclear risk of bias specifically concerning the blinding of personnel and assessors. Studies by Carlson et al. (2015), Duraimani et al. (2015), and Ho et al. (2012) had overall high risks of bias. The study of Le Nguyen et al. (2019) was the only one with an overall low risk of bias. The quasi-experimental study of Conklin et al. (2018) had unclear risks of bias (Figure S6). Of the four case–control studies, two had an overall low risk of bias, one unclear risk of bias, and one high risk of bias (Figures S7 and S8): Krishna et al. (2015) did not provide information about how confounds were identified and which strategies were used to address them.
As the Joanna Briggs Institute checklists do not include an overall risk of bias rating, we used the same approach as the Cochrane risk of bias tool for RCTs (Higgins & Altmann, 2008) to arrive at an overall rating: studies that included at least one domain with an unclear or high risk of bias were rated to have overall unclear or high risk of bias, respectively. Only studies with a low risk of bias in every domain were rated to have an overall low risk of bias. Across the entire study pool, only three studies were rated with an overall low risk of bias. For 17 studies, there was an unclear risk of bias, whereas four studies had an overall high risk of bias.
TL and TA Summary Effects
The analysis revealed a small summary effect of MBIs on TL (g = 0.23, SE = 0.08, 95% CI = [0.07, 0.39], p = 0.01). On average, participants’ telomeres were 0.23 standard deviations longer in the MBI conditions than in the control conditions. The Q test revealed significant effect-size heterogeneity (Q = 44.54, df = 19, p < 0.001), indicating that studies did not share a common effect size. According to the I2 statistics, 0% of the observed effect variance stemmed from variance in the true effects between studies, whereas 60% was from within studies (for studies with more than one intervention group). The 95% prediction interval was [− 0.35, 0.80], indicating a large variation of effects and directionally opposing effects.
The summary effect of MBIs on TA was small to moderate (g = 0.37, SE = 0.18, 95% CI = [0.01, 0.73], p = 0.05). On average, participants’ telomerase activity was 0.37 standard deviations higher in the MBIs conditions than in the control conditions. The studies did not share a common effect size (Q = 78.99, df = 13, p < 0.01). Eighty-four percent of the observed effect variance stemmed from variance in the true effects between studies, whereas 0% was from within studies. The 95% prediction interval [− 1.02, 1.75] indicated again a large variation of effects and directionally opposing effects.
Risk of Bias Across Studies
Sunset funnel plots for studies assessing TL and TA are displayed in Fig. 1. Visually, there appeared to be an asymmetry in the distribution of effect sizes for TL, but not TA. The median analytic power of the studies to detect the observed summary effects (excluding one study for TL, see below) was 14% for TL and 21% for TA, respectively. The expected replicability of findings (R-index) was 3% for TL and 0% for TA. The tests of excess significance (TES) for TL and TA, computed by hand for the latter, yielded non-significant results (TL: observed = 5, expected = 3.53, p = 0.39; TA: observed = 5, expected = 5.59, p = 1.00).
Outliers and Moderators
Outlier analyses revealed no outliers for TL and TA. Interpreting reported SDs as SEs, the study of Krishna et al. (2015), which appeared to be an outlier in Schutte et al. (2020; g = 5.59), did not provide a conspicuously large effect anymore (g = 1.34).
As effects were heterogeneous across studies, we conducted meta-regression analyses for both TL (Table 3) and TA (Table 4). A number of moderators were nominally significant (p < 0.05) for TL: effects were significant only in studies that (1) reported no conflicts of interest (Table 3; other studies: g = − 0.07, SE = 0.15, 95% CI = [− 0.36, 0.21], p = 0.62); (2) investigated experienced meditators (Table 3; other studies combined: g = 0.11, SE = 0.07, 95% CI = [− 0.04, 0.24], p = 0.14); and (3) were not conducted as RCTs (g = 0.52, SE = 0.15, 95% CI = [0.22, 0.82], p < 0.01; see Table 3 for RCTs). Furthermore, effects (4) decreased with study n (Fig. 2, left panel), which is indicative of small-study effects and, hence, publication bias, and (5) increased with meditation dose (Fig. 2, right panel).
For TA, three moderators were nominally significant: effects were significant only in studies (1) that reported no conflicts of interest (Table 4; other studies: g = − 0.08, SE = 0.19, 95% CI = [− 0.46, 0.30], p = 0.66) and (2) compared MBIs to no interventions only (Table 4; other studies: g = − 0.16, SE = 0.18, 95% CI = [− 0.88, 0.38], p = 0.38); furthermore, effects were significant (3) only in studies from Asia (g = 0.93, SE = 0.22, 95% CI = [0.50, 1.36], p < 0.01; see Table 4 for other regions).
The final model of the random forest analysis for TL explained 22% of the variance in new data (“out-of-bag,” which means that data were predicted by trees that were trained on bootstrap samples not containing that data; van Lissa, 2020b). The relative importance of included moderators for telomere length is displayed in Figure S9 (left): dosage was the most important moderator, followed by intervention elements, study n, and study design. Together, these moderators specifically appeared to characterize the four studies of Alda et al. (2016), Hoge et al. (2013), Krishna et al. (2015), and Mendioroz et al. (2020) that compared experienced meditators with no-intervention controls in retrospective case–control designs, and which together had a large summary effect (Table 3), compared to all other studies (Table 3 and above), for which no significant summary effect was observed. In the final model for TA, control condition and region explained 7% of the variance in new data (“out-of-bag”; Figure S9, right).
Discussion
The present study synthesized the currently available evidence from randomized and non-randomized studies on the effects of MBIs on telomere length and telomerase activity, drawing on a substantially increased study pool than previous meta-analyses and testing a large number of possible effect moderators. We observed small-to-medium summary effects (g = 0.23 and 0.37) of MBIs on TL and TA (RQ1 and RQ2). However, these effects appeared to be driven either by retrospective case–control studies with meditators with long years of experience (TL) or by studies without control interventions and studies from Asia (TA) (RQ3). Study quality and analytic power to detect the current meta-analytic summary effects appeared to be low among the available primary studies.
The small summary effect of MBIs on TL is in good accordance with and is even slightly larger than in, previous meta-analyses (Dunn & Dimolareva, 2022; Schutte et al., 2020). However, the much larger summary effect for TA reported in previous meta-studies (Dunn & Dimolareva, 2022; Schutte and Malouff, 2014) could not be replicated in the present meta-analysis. Notably, previous meta-analyses synthesized the data of only 4 and 7 studies on TA, whereas the present results were based on the data of 13 studies (and 14 effect sizes within these studies). Thus, the available evidence suggests that the effects of MBIs on TA are, on average, smaller than previously assumed and may not exceed similar effects of other active interventions.
It is known that cloistered monks and nuns have a lower mortality risk, and thus live longer, than the overall population (e.g., Luy, 2003; evidence for this can be traced back even to medieval European populations; see DeWitte et al., 2013). Studies by Alda et al. (2016), Hoge et al. (2013), Krishna et al. (2015), and Mendioroz et al. (2020) all investigated meditators with years-long intensive meditation experience. Even though participants in these studies might not be directly comparable to nuns or monks, it is conceivable that some of the factors driving longevity in nuns and monks might also apply to them (e.g., a higher educational background, but also lifestyle factors associated with better nutrition and lower levels of social stress). This may need further study in the future (note that Alda et al., 2016, and Hoge et al., 2013, actually matched meditators and non-meditators in their studies by age, education, and lifestyle factors, such as physical exercise, smoking, body-mass index, and diet). However, judging from the currently available evidence, an increase in TL may not be expected with MBIs in groups with less or even only brief meditation experience. This is consistent with the latest neuroscientific data, which suggest that brief mindfulness interventions (i.e., involving less than 27 hr of meditation) are also not enough to cause measurable changes in amygdala volume (Kral et al., 2022). Meditation may need to be performed over longer periods to result also in discernable effects on the cellular level of TL. Yet, even then, its effects could still be (partly) confounded with other causal factors, for which meditation is only a proxy. Thus, TL might not provide a useful outcome to assess the efficacy of common (brief) MBIs or related interventions.
Concerning TA, more studies with active control conditions are needed to draw firmer conclusions on the potential specificity of the effects of MBIs. Currently, there is no evidence that MBIs may have specific effects on TA, which could not be similarly achieved with other active interventions as well. Yet, to the extent that MBIs do appear to increase TA, it could be used as an objective measure for the efficacy of mindfulness interventions. It could complement, or replace, more subjective self-ratings of psychometric mindfulness or mental health in this field of research (Goldberg et al., 2022; Tran et al., 2022). This needs to be addressed in future research, which also needs to investigate in more detail the exact conditions under which either high or low telomerase levels can be considered a marker for good or improved cellular health (Epel, 2012).
Limitations and Future Directions
This study presented the to-date most comprehensive meta-analysis on the topic of MBIs and TL and TA. However, concerns regarding the risk of bias in primary studies and publication bias suggested that the current meta-analytic summary effects still may have been overestimated and the utilized search strategy may have been suboptimal in retrieving non-English studies as only the first 500 hits in Google Scholar were screened for eligibility in the second stage of the literature search. Study quality needs to be increased in this field of research (see also Goldberg et al., 2022; Tran et al., 2022) and there is a need for pre-registration and open data to increase the statistical conclusion validity of reported results. Puhlmann et al. (2019) reported large individual differences in TL changes. Open data are needed here to examine such differences on the individual level or with individual participant-level data meta-analysis. Observed larger effects in studies, which reported conflicts of interest, versus studies, which did not, may hint at further transparency problems of available studies. Larger samples are needed to increase studies’ analytic power.
Active control conditions covered a variety of interventions, but only in small minority interventions that could be deemed fully psychotherapeutic (e.g., CBT). More transparent, high-quality, and high-powered studies with psychotherapeutic control interventions are needed to gain insight into whether the effects of MBIs on TA are specific to them or generalize also to other psychotherapeutic interventions. Also, more studies from Europe and the US are needed to ascertain the observed effects of MBIs on TA. Future RCT studies should investigate the effects of meditation on TL over longer time periods and control also for potential confounding factors. The utility of TA as an objective measure in intervention studies needs to be probed.
Data Availability
The preregistration and all data, materials, and code to reproduce the analysis are available at the Open Science Framework https://osf.io/827uk/
References
*Studies which were included in the meta-analysis
*Alda, M., Puebla-Guedea, M., Rodero, B., Demarzo, M., Montero-Marin, J., Roca, M., & Garcia-Campayo, J. (2016). Zen meditation, length of telomeres, and the role of experiential avoidance and compassion. Mindfulness, 7(3), 651–659. https://doi.org/10.1007/s12671-016-0500-5
Black, D. S., & Slavich, G. M. (2016). Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Annals of the New York Academy of Science, 1373, 13–24. https://doi.org/10.1111/nyas.12998
Blackburn, E. H., Epel, E. S., & Lin, J. (2015). Human telomere biology: A contributory and interactive factor in aging, disease, and protection. Science, 350(6265), 1193–1198. https://doi.org/10.1126/science.aab3389
Bodhi, B. (2011). What does mindfulness really mean? A Canonical Perspective. Contemporary Buddhism, 12(1), 19–39. https://doi.org/10.1080/14639947.2011.564813
Bojesen, S. E. (2013). Telomeres and human health. Journal of Internal Medicine, 274(5), 399–413. https://doi.org/10.1111/joim.12083
Borenstein, M., Higgins, J. P. T., Hedges, L. V., & Rothstein, H. R. (2017). Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Research Synthesis Methods, 8(1), 5–18. https://doi.org/10.1002/jrsm.1230
Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2021). Introduction to meta-analysis (2nd ed.). Wiley.
*Carlson, L. E., Beattie, T. L., Giese-Davis, J., Faris, P., Tamagawa, R., Fick, L. J., Degelman, E. S., & Speca, M. (2015). Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer, 121(3), 476–484. https://doi.org/10.1002/cncr.29063
Carter, E. C., Schönbrodt, F. D., Gervais, W. M., & Hilgard, J. (2019). Correcting for bias in psychology: A comparison of meta-analytic methods. Advances in Methods and Practices in Psychological Science, 2(2), 115–144. https://doi.org/10.1177/2515245919847196
Chan, S. R. W. L., & Blackburn, E. H. (2004). Telomeres and telomerase. Philosophical Transactions of the Royal Society, 359(1441), 109–122. https://doi.org/10.1098/rstb.2003.1370
Chiesa, A., Anselmi, R., & Serretti, A. (2014). Psychological mechanisms of mindfulness-based stress interventions. Holistic Nursing Practice, 28(2), 124–148. https://doi.org/10.1097/HNP.0000000000000017
*Conklin, Q. A., King, B. G., Zanesco, A. P., Lin, J., Hamidi, A. B., Pokorny, J. J., Álvarez-López, M. J., Cosín-Thomás, M., Huang, C., Kaliman, P., Epel, E. S., & Saron, C. D. (2018). Insight meditation and telomere biology: The effects of intensive retreat and the moderating role of personality. Brain, Behavior, and Immunity, 70, 233–245. https://doi.org/10.1016/j.bbi.2018.03.003
Dasanayaka, N. N., Sirisena, N. D., & Samaranayake, N. (2021). The effects of meditation on length of telomeres in healthy individuals: A systematic review. Systematic Reviews, 10, 151. https://doi.org/10.1186/s13643-021-01699-1
*Dasanayaka, N. N., Sirisena, N. D., & Samaranayake, N. (2022). Impact of meditation-based lifestyle practices on mindfulness, wellbeing, and plasma telomerase levels: A case-control study. Frontiers in Psychology, 13, Article 846085. https://doi.org/10.3389/fpsyg.2022.846085
*Daubenmier, J., Lun, J., Blackburn, E., Hecht, F. M., Kristeller, J., Maninger, N., Kuwata, M., Bacchetti, P., Havel, P. J., & Epel, E. (2012). Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology, 37(7), 917–928. https://doi.org/10.1016/j.psyneuen.2011.10.008
DeWitte, S. N., Boulware, J. C., & Redfern, R. C. (2013). Medieval monastic mortality: Hazard analysis of mortality differences between monastic and nonmonastic cemeteries in England. American Journal of Biological Anthropology, 152(3), 322–332. https://doi.org/10.1002/ajpa.22350
*Duan, G.-X., Wang, K., Su, Y.-H., Tang, S.-Y., Jia, H.-L., Chen, X.-M., & Xie, H.-H. (2016). Effects of Tai Chi on telomerase activity and gerotranscendence in middle aged and elderly adults in Chinese society. International Journal of Nursing Sciences, 3(3), 235–241. https://doi.org/10.1016/j.ijnss.2016.07.005
Dunn, T. J., & Dimolareva, M. (2022). The effect of mindfulness-based interventions on immunity-related biomarkers: A comprehensive meta-analysis of randomised controlled trials. Clinical Psychology Review, 92, 102124. https://doi.org/10.1016/j.cpr.2022.102124
*Duraimani, S., Schneider, R. H., Randall, O. S., Nidich, S. I., Xu, S., Ketete, M., Rainforth, M. A., Gaylord-King, C., Salerno, J. W., & Fagan, J. (2015). Effects of lifestyle modification on telomerase gene expression in hypertensive patients: A pilot trial of stress reduction and health education programs in African Americans. PLoS ONE, 10(11), e0142689. https://doi.org/10.1371/journal.pone.0142689
Epel, E. (2012). How “reversible” is telomeric aging? Cancer Prevention Research, 5(10), 1163–1168. https://doi.org/10.1158/1940-6207.CAPR-12-0370
Epel, E. S., & Prather, A. A. (2018). Stress, telomeres, and psychopathology: Toward a deeper understanding of a triad of early aging. Annual Review of Clinical Psychology, 14(1), 371–397. https://doi.org/10.1146/annurev-clinpsy-032816-045054
Epel, E., Daubenmier, J., Moskowitz, J. T., Folkman, S., & Blackburn, E. (2009). Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Longevity, Regeneration, and Optimal Health, 1172(1), 34–53. https://doi.org/10.1111/j.1749-6632.2009.04414.x
*Epel, E. S., Puterman, E., Lin, J., Blackburn, E. H., Lum, P. Y., Beckmann, N. D., Zhu, J., Lee, E., Gilbert, A., Rissman, R. A., Tanzi, R. E., & Schadt, E. E. (2016). Meditation and vacation effects have an impact on disease-associated molecular phenotypes. Translational Psychiatry, 6, e880. https://doi.org/10.1038/tp.2016.164
*Gardener, C. M. (2018). Exploring the effects of acceptance and commitment therapy (ACT) on biomarkers of stress in breast cancer survivors [Bachelor of Science thesis, University of Southern Queensland]. USQ ePrints. http://eprints.usq.edu.au/id/eprint/35883
*Gautam, S., Tolahunase, M., Kumar, U., & Dada, R. (2019). Impact of yoga based mind-body intervention on systemic inflammatory markers and co-morbid depression in active rheumatoid arthritis patients: A randomized controlled trial. Restorative Neurology and Neuroscience, 37(1), 41–59. https://doi.org/10.3233/RNN-180875
Goldberg, S. B., Tucker, R. P., Greene, P. A., Simpson, T. L., Hoyt, W. T., Kearney, D. J., & Davidson, R. D. (2019). What can we learn from randomized clinical trials about the construct validity of self-report measures of mindfulness? A meta-analysis. Mindfulness, 10(5), 775–785. https://doi.org/10.1007/s12671-018-1032-y
Goldberg, S. B., Riordan, K. M., Sun, S., & Davidson, R. J. (2022). The empirical status of mindfulness-based interventions: A systematic review of 44 meta-analyses of randomized controlled trials. Perspectives on Psychological Science, 17(1), 108–130. https://doi.org/10.1177/1745691620968771
Hayes, S. C., Strosahl, K., & Wilson, K. G. (1999). Acceptance and Commitment Therapy: An experiential approach to behavior change. Guilford Press.
*Hendrich, S. G. (2019). Effects of a mindfulness-based intervention on depressive symptoms, stress eating, and telomere length in adolescents at-risk for adult obesity (Publication No. 13897114) [Master of Science thesis, Colorado State University]. ProQuest LLC.
Higgins, J. P. T., & Altmann, D. G. (2008). Assessing risk of bias in included studies. In J. P. T. Higgins & S. Green (Eds.), Cochrane handbook for systematic reviews of interventions (pp. 187–241). Wiley. https://doi.org/10.1002/9780470712184
*Ho, R. T. H., Chan, J. S. M., Wang, C.-W., Lau, B. W. M., So, K. F., Yuen, L. P., Sham, J. S. T., & Chan, C. L. W. (2012). A randomized controlled trial of qigong exercise on fatigue symptoms, functioning, and telomerase activity in persons with chronic fatigue or chronic fatigue syndrome. Annals of Behavioral Medicine, 44(2), 160–170. https://doi.org/10.1007/s12160-012-9381-6
*Hoge, E. A., Chen, M. M., Orr, E., Metcalf, A., Fischer, L. E., Pollack, M. H., DeVivo, I., & Simon, N. M. (2013). Loving-kindness meditation practice associated with longer telomeres in women. Brain, Behavior, and Immunity, 32, 159–163. https://doi.org/10.1016/j.bbi.2013.04.005
Hölzel, B. K., Lazar, S. W., Gard, T., Schuman-Olivier, Z., Vago, D. R., & Ott, U. (2011). How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science, 6(6), 537–559. https://doi.org/10.1177/1745691611419671
*Innes, K. E., Selfe, T. K., Brundage, K., Montgomery, C., Wen, S., Kandati, S., Bowles, H., Khalsa, D., & Huysmans, Z. (2018). Effects of meditation and music-listening on blood biomarkers of cellular aging and Alzheimer’s disease in adults with subjective cognitive decline: An exploratory randomized clinical trial. Journal of Alzheimer’s Disease, 66(3), 947–970. https://doi.org/10.3233/JAD-180164
Ioannidis, J. P., & Trikalinos, T. A. (2007). An exploratory test for an excess of significant findings. Clinical Trials, 4, 245–253. https://doi.org/10.1177/1740774507079441
*Jacobs, T. L., Epel, E. S., Lin, J., Blackburn, E. H., Wolkowitz, O. M., Bridwell, D. A., Zanesco, A. P., Aichele, S. R., Sahdra, B. K., MacLean, K. A., King, B. G., Shaver, P. R., Rosenberg, E. L., Ferrer, E., Wallace, A. B., & Saron, C. D. (2011). Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology, 36, 664–681. https://doi.org/10.1016/j.psyneuen.2010.09.010
Kabat-Zinn, J. (1990). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Random House LLC.
*Keng, S.-L., Looi, P. L., Tan, E. L. Y., Yim, O.-S., Lai, P. S., Chew, S. H., & Ebstein, R. P. (2020). Effects of mindfulness-based stress reduction on psychological symptoms and telomere length: A randomized active-controlled trial. Behavior Therapy, 51(6), 984–996. https://doi.org/10.1016/j.beth.2020.01.005
Kossmeier, M., Tran, U. S., & Voracek, M. (2020b). Power-enhanced funnel plots for meta-analysis: The sunset funnel plot. Zeitschrift Für Psychologie, 228(1), 43–49. https://doi.org/10.1027/2151-2604/a000392
Kossmeier, M., Tran, U. S, & Voracek, M. (2020a). Metaviz: Forest plots, funnel plots, and visual funnel plot inference for meta-analysis (Version 0.3.1) [Computer software]. https://cran.r-project.org/web/packages/metaviz/index.html
Kral, T. R. A., Davis, K., Korponay, C., Hirshberg, M. J., Hoel, R., Tello, L. Y., Goldman, R. I., Rosenkranz, M. A., Lutz, A., & Davidson, R. J. (2022). Absence of structural brain changes from mindfulness-based stress reduction: Two combined randomized controlled trials. Science Advances, 8(20), eabk316. https://doi.org/10.1126/sciadv.abk3316
*Krishna, B. H., Keerthi, G. S., Kumar, C. K., & Reddy, N. M. (2015). Association of leukocyte telomere length with oxidative stress in yoga practitioners. Journal of Clinical and Diagnostic Research, 9(3), CC01–CC03. https://doi.org/10.7860/JCDR/2015/13076.5729
*Lavretsky, H., Siddarth, P., Nazarian, N., Cyr, N. St., Khalsa, D. S., Lin, J., Blackburn, E., Epel, E. S., & Irwin, M. R. (2013). A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: Effects on mental health, cognition, and telomerase activity. International Journal of Geriatric Psychiatry, 28(1), 57–65. https://doi.org/10.1002/gps.3790
*Le Nguyen, K. D., Lin, J., Algoe, S. B., Brantley, M. M., Kim, S. L., Brantley, J., Salzberg, S., & Fredrickson, B. L. (2019). Loving-kindness meditation slows biological aging in novices: Evidence from a 12-week randomized controlled trial. Psychoneuroendocrinology, 108, 20–27. https://doi.org/10.1016/j.psyneuen.2019.05.020
*Lengacher, C. A., Reich, R. R., Kip, K. E., Barta, M., Ramesar, S., Paterson, C. L., Moscoso, M. S., Carranza, I., Budhrani, P. H., Kim, S. J., Park, H. Y., Jacobsen, P. B., Schell, M. J., Jim, H. S. L., Post-White, J., Farias, J. R., & Park, J. Y. (2014). Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in woman with breast cancer (BC). Biological Research for Nursing, 16(4), 438–447. https://doi.org/10.1177/1099800413519495
Lin, J., Epel, E., & Blackburn, E. (2012). Telomeres and lifestyle factors: Roles in cellular aging. Mutation Research, 730(1–2), 85–89. https://doi.org/10.1016/j.mrfmmm.2011.08.003
Lindsay, E. K., & Creswell, J. D. (2017). Mechanisms of mindfulness training: Monitor and Acceptance Theory (MAT). Clinical Psychology Review, 51, 48–59. https://doi.org/10.1016/j.cpr.2016.10.011
Luy, M. (2003). Causes of male excess mortality: Insights from cloistered populations. Population and Development Review, 29(4), 647–676. https://doi.org/10.1111/j.1728-4457.2003.00647.x
*Mason, A. E., Hecht, F. M., Daubenmier, J. J., Sbarra, D. A., Lin, J., Moran, P. J., Schleicher, S. G., Acree, M., Prather, A. A., & Epel, E. S. (2018). Weight loss, weight-loss maintenance, and cellular aging in the supporting health through nutrition and exercise (SHINE) study. Psychosomatic Medicine, 80(7), 609–619. https://doi.org/10.1097/PSY.0000000000000616
*Mendioroz, M., Puebla-Guedea, M. P., Montero-Marín, J., Urdánoz-Casado, A., Blanco-Luquin, I., Roldán, M., Labarga, A., & García-Campayo, J. (2020). Telomere length correlates with subtelomeric DNA methylation in long-term mindfulness practitioners. Scientific Reports, 10, 4564. https://doi.org/10.1038/s41598-020-61241-6
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., The PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLOS Medicine, 6(7), 1000097. https://doi.org/10.1371/journal.pmed.1000097
Moola, S., Munn, Z., Tufanaru, C., Aromataris, E., Sears, K., Sfetcu, R., Currie, M., Lisy, K., Qureshi, R., Mattis, P., & Mu, P. (2020). Systematic reviews of etiology and risk. In E. Aromataris, Z. Munn (Eds.), JBI manual for evidence synthesis. JBI. https://synthesismanual.jbi.global
Morris, S. C. (2008). Estimating effect sizes from pretest-posttest-control group designs. Organizational Research Methods, 11(2), 364–386. https://doi.org/10.1177/1094428106291059
Munn, Z., Moola, S., Lisy, K., Riitano, D., Sears, K., & Tufanaru, C. (2020). Systematic reviews of effectiveness. In E. Aromataris, Z. Munn (Eds.), JBI manual for evidence synthesis. JBI. https://synthesismanual.jbi.global
O’Connor, D., Green, S., & Higgins, J. P. T. (2008). Defining the review question and developing criteria for including studies. In J. P. T. Higgins & S. Green (Eds.), Cochrane handbook for systematic reviews of interventions (pp. 81–94). Wiley. https://doi.org/10.1002/9780470712184
Protzko, J., & Schooler, J. W. (2017). Decline effects: Types, mechanisms, and personal reflections. In S. O. Lilienfeld and I. D. Waldman (Eds.), Psychological science under scrutiny: Recent challenges and proposed solutions (pp. 85–107). Wiley. https://doi.org/10.1002/9781119095910.ch6
*Puhlmann, L. M. C., Valk, S. L., Engert, V., Bernhardt, B. C., Lin, J., Epel, E. S., Vrtička, P., & Singer, T. (2019). Association of short-term change in leukocyte telomere length with cortical thickness and outcomes of mental training among healthy adults. A randomized clinical trial. JAMA Network Open, 2(9), e199687. https://doi.org/10.1001/jamanetworkopen.2019.9687
Putterman, E., & Epel, E. (2012). An intricate dance: Life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Social and Personality Psychology Compass, 6(11), 807–825. https://doi.org/10.1111/j.1751-9004.2012.00465.x
Schimmack, U. (2016). The replicability-index: Quantifying statistical research integrity. Retrieved from https://www.replicationindex.
Schutte, N. S., & Malouff, J. M. (2014). A meta-analytic review of the effects of mindfulness meditation on telomerase activity. Psychoneuroendocrinology, 42, 45–48. https://doi.org/10.1016/j.psyneuen.2013.12.017
Schutte, N. S., Malouff, J. M., & Keng, S. (2020). Meditation and telomere length: A meta-analysis. Psychology & Health, 35(8), 901–915. https://doi.org/10.1080/08870446.2019.1707827
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., Shekelle, P., & Stewart, L. A. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ, 349, g7647. https://doi.org/10.1136/bmj.g7647
*Tolahunase, M. R., Sagar, R., Faiq, M., & Dada, R. (2018). Yoga- and meditation-based lifestyle intervention increases neuroplasticity and reduces severity of major depressive disorder: A randomized controlled trial. Restorative Neurology and Neuroscience, 36(3), 423–442. https://doi.org/10.3233/RNN-170810
Tran, U. S., Birnbaum, L., Burzler, M. A., Hegewisch, U. J. C., Ramazanova, D., & Voracek, M. (2022). Self-reported mindfulness accounts for the effects of mindfulness interventions and nonmindfulness controls on self-reported mental health: A preregistered systematic review and three-level meta-analysis of 146 randomized controlled trials. Psychological Bulletin, 148(1–2), 86–106. https://doi.org/10.1037/bul0000359
Vago, D. R., & Silbersweig, D. A. (2012). Self-awareness, self-regulation, and self-transcendence (S-ART): A framework for understanding the neurobiological mechanisms of mindfulness. Frontiers in Human Neuroscience, 6, 296. https://doi.org/10.3389/fnhum.2012.00296
Van Dam, N. T., Van Vugt, M. K., Vago, D. R., Schmalzl, L., Saron, C. D., Olendzki, A., Meissner, T., Lazar, S. W., Kerr, C. E., Gorchov, J., Fox, K. C. R., Field, B. A., Britton, W. B., Brefczynski-Lewis, J. A., & Meyer, D. E. (2018). Mind the hype: A critical evaluation and prescriptive agenda for research on mindfulness and meditation. Perspectives on Psychological Science, 13(1), 36–61. https://doi.org/10.1177/1745691617709589
van Lissa, C. J. (2020a). Metaforest: Exploring heterogeneity in meta-analysis using random forests (version 0.1.3) [R-package]. https://cran.r-project.org/web/packages/metaforest/index.html
van Lissa, C. J. (2020b). Small sample meta-analyses: Exploring heterogeneity using MetaForest. In R. Van De Schoot & M. Miočević (Eds.), Small sample size solutions: A guide for applied researchers and practitioners (pp. 186–202). Routledge. https://www.routledge.com/Small-Sample-Size-Solutions-Open-Access-A-Guide-for-Applied-Researchers/Schoot-Miocevic/p/book/9780367222222
Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. https://doi.org/10.18637/jss.v036.i03
Viechtbauer, W. (n.d.-a). Some recommended practices. https://wviechtb.github.io/metafor/reference/misc-recs.html
Viechtbauer, W. (n.d.-b). Construct or approximate the variance-covariance matrix of dependent effect sizes or outcomes. https://wviechtb.github.io/metafor/reference/vcalc.html
*Wang, X., Sundquist, K., Hedelius, A., Palmér, K., Memon, A. A., & Sundquist, J. (2017). Leukocyte telomere length and depression, anxiety and stress and adjustment disorders in primary health care patients. BMC Psychiatry, 17, Article 148. https://doi.org/10.1186/s12888-017-1308-0
Funding
Open access funding provided by University of Vienna. Open-access funding is provided by the University of Vienna.
Author information
Authors and Affiliations
Contributions
Lukas Bossert: conceptualization, data curation, formal analysis, methodology, visualization, writing—original draft, writing—review and editing. Karla Arzberger: conceptualization, data curation, investigation, methodology, writing—review and editing. Fabian Dorok: conceptualization, investigation, methodology, writing—original draft, writing—review and editing. Julia Kern: conceptualization, data curation, investigation, methodology, writing—review and editing. Charlotte Stickler: conceptualization, data curation, methodology, writing—review and editing. Maximilian Wunderlich: conceptualization, data curation, investigation, methodology, writing—review and editing. Ulrich S. Tran: conceptualization, methodology, project administration, supervision, validation, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Ethics Statement
The manuscript does not contain individual participant or patient data.
Informed Consent Statement
None required.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bossert, L., Arzberger, K., Dorok, F. et al. The Effects of Mindfulness-Based Interventions on Telomere Length and Telomerase Activity: A Systematic Review and Meta-Analysis. Mindfulness 14, 495–509 (2023). https://doi.org/10.1007/s12671-023-02075-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12671-023-02075-x