Abstract
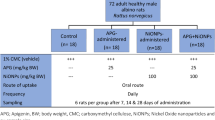
Despite the documented adverse impacts of nanoparticles (NPs) on the environment and human health, their application in nanomedicine is expanding. Because nickel compounds are very toxic to the liver and kidney and can cause cancer, it is important to research how they affect human health. The effects of spherical nickel nanoparticles (Ni-NPs) on kidney and liver functions were demonstrated in the current investigation. Twenty male albino rats were separated into two groups. The first group was used as a control group, which received only 0.9% sodium chloride, while the other group II, the rats, were orally administered Ni-NPs at a concentration of 50 mg/kg body weight by gavage three times a week for 4 consecutive weeks got. The animal’s blood, liver, and kidney samples were taken and analyzed for cell structure and function. The nephrotoxic effect of Ni-NPs and its correlation to the oxidative state have been studied using standard diagnostic techniques such as biochemical testing and histopathology. Tissue biochemical analysis of malondialdehyde (MDA), catalase (CAT), reduced glutathione (GSH), and superoxide dismutase (SOD) was recorded in the liver and kidney. The Ni-NP-treated group refers to the impaired kidney functions resulting from deposit of Ni-NPs in the kidney and the oxidative stress of the liver. Ni-NPs induce changes in histopathology and ultrastructure in liver and kidney tissue. The conductivity has much higher values for the treated group compared with the control group; this is an indicator that Ni-NPs are affected in the blood condition in which blood hemolysis occurs, consequently anemic disease. Ni-NPs induce DNA damage through generation of reactive oxidative stress (ROS), nephrotoxic effects, lipid peroxidation, and inflammation, and also induced an increase in blood viscosity, showing platelet clumps, hepatotoxicity, inflammation, fatty changes in liver, and histopathological changes in liver and kidney tissue.












Similar content being viewed by others
Availability of Data and Materials
The authors declare that all relevant data of this study are included in the article.
Abbreviations
- Ni-NPs:
-
Nickel nanoparticles
- MDA:
-
Malondialdehyde
- CAT:
-
Catalase
- GSH:
-
Glutathione peroxidase
- SOD:
-
Superoxide dismutase
- DNA:
-
Deoxyribonucleic acid
- PT:
-
Plasma tubule cells
- ROS:
-
Reactive oxygen species
- TEM:
-
Transmission electron microscopy
- 8-OHDG:
-
8-Hydroxyl-2doxyguanosine
- 5-NT:
-
5-Nucleotidase
- RBCs:
-
Red blood cells
- CV:
-
Central vein
- BS:
-
Blood sinusoids
- LI:
-
Leucocyte infiltration
- BD:
-
Bile ductules
- P:
-
Pyknotic
- KH:
-
Karyorrhectic
- BS:
-
Blood sinusoid
- KC:
-
Kupffer cells
- NF-KB:
-
Nuclear factor kappa B
References
Hoeijmakers, J. H. J. (2012). The key role of DNA damage on cancer, aging and longevity. Environ. Mol. Mutagenesis, 53, S13. https://doi.org/10.1093/carcin/bgaa114
Schiewer, M. J., & Knudsen, K. E. (2019). DNA damage response in prostate cancer. CSH Perspect. Med, 9, a030486. https://doi.org/10.1101/cshperspect.a030486
Lord, C. J., & Ashworth, A. (2012). The DNA damage response and cancer therapy. Nature, 481, 287–294. https://doi.org/10.1038/nature10760
Basu, A. K. (2018). DNA damage, mutagenesis and cancer. International Journal of Molecular Sciences, 19, 970. https://doi.org/10.3390/ijms19040970
Hoeijmakers, J. H. J. (2009). DNA damage, aging, and cancer. New England Journal of Medicine, 361, 1914. https://doi.org/10.1093/carcin/bgaa114
Doll, R. (1984). Nickel exposure: A human health hazard. IARC Scientific Publications, 53, 3–21.
Nali, T., Salmani, F., & Naseri, K. (2019). Dietary intake of cadmium, chromium, copper, nickel, and lead through the consumption of meat, liver, and kidney and assessment of human health risk in birjand, southeast of Iran. Biological Trace Element Research, 191, 338–347. https://doi.org/10.1007/s12011-019-1637-6
Haber, L. T., Erdreicht, L., Diamond, G. L., Maier, A. M., et al. (2000). Hazard identification and dose response of inhaled nickel-soluble salts. Regul Toxicol Pharmacol, 31, 210–230. https://doi.org/10.1006/rtph.2000.1377
Das, K. K., Das, S. N., & Dhundasi, S. A. (2008). Nickel, its adverse health effects & oxidative stress. Indian J. Med. Res, 128, 412–425. https://doi.org/10.2174/0929867053764635
Nakamura, H., & Watano, S. (2018). Direct permeation of nanoparticles across cell membrane: A review. KONA Powder and Particle Journal, 35, 49–65. https://doi.org/10.14356/kona.2018011
Chen, P., Zhang, Z., Xing, J., Gu, N., & Ji, M. (2017). Physicochemical properties of nanoparticles affect translocation across pulmonary surfactant monolayer. Molecular Physics, 115, 3143–3154. https://doi.org/10.1080/00268976.2017.1351005
Ding, D., Liu, K., Fan, Q., et al. (2018). Nickel nanoparticles individually encapsulated in densified ceramic shells for thermally stable solar energy absorption. J Mater Chem A, 7, 3039–3045. https://doi.org/10.1039/C8TA10690H
Shahzad, R., Waqas, M., Khan, A. L., Asaf, S., et al. (2016). Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Phymsiology and Biochemistry, 106, 236–243. https://doi.org/10.1016/j.plaphy.2016.05.006
Zambelli, B., Uversky, V. N., & Ciurli, S. (2016). Nickel impact on human health: An intrinsic disorder perspective. Biochimica et Biophysica Acta, 1864(12), 1714–1731. https://doi.org/10.1016/j.bbapap.2016.09.008
Salnikow, K., & Zhitkovich, A. (2008). Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis:Nickel, arsenic, and chromium. Chemical Research in Toxicology, 21, 28–44. https://doi.org/10.1021/tx700198a
Nakamura, H., & Watano, S. (2018). Direct permeation of nanoparticles across cell membrane: A review. KONA Powder Particle Journal, 35, 49–65. https://doi.org/10.14356/kona.2018011
Zhang, Q., Kusaka, Y., Zhu, X., Sato, K., Mo, et al. (2003). Comparative toxicity of standard nickel and ultrafine nickel in lung after intratracheal instillation. J. Occup. Health, 45(1), 23–30. https://doi.org/10.1539/joh.45.23
Sivulka, D. J. (2005). Assessment of respiratory carcinogenicity associated with exposure to metallic nickel: A review. Regulatory Toxicology and Pharmacology, 43, 117–133. https://doi.org/10.1016/j.yrtph.2005.06.014
Ma, C., Songb, M., Zhang, Y., et al. (2014). Nickel nanowires induce cell cycle arrest and apoptosis by generation of reactive oxygen species in HeLa cells. Toxicology Reports, 1, 114–121. https://doi.org/10.1016/j.toxrep.2014.04.008
Magaye, R. R., Yue, X., Zou, B., Shi, H., et al. (2014). Acute toxicity of nickel nanoparticles in rats after intravenous injection. Int J Nanomed, 9, 1393–1402. https://doi.org/10.2147/IJN.S56212
Magaye, R., Zhou, Q., Bowman, L., Zou, B., et al. (2014). Metallic nickel nanoparticles may exhibit higher carcinogenic potential than fine particles in JB6 cells. PLoS One, 9(4), e92418. https://doi.org/10.1371/journal.pone.0092418
Stine, J. G., & Lewis, J. H. (2016). Current and future directions in the treatment and prevention of drug-induced liver injury: A systematic review. Expert Review of Gastroenterology & Hepatology, 10(4), 517–536. https://doi.org/10.1586/17474124.2016.1127756
Pari, L., & Prasath, A. (2008). Efficacy of caffeic acid in preventing nickel induced oxidative damage in liver of rats. Chem Biol Interact, 173, 77–83. https://doi.org/10.1016/j.cbi.2008.02.010
Abdulqadir, S. Z., & Aziz, F. M. (2019). Internalization and effects on cellular ultrastructure of nickel nanoparticles in rat kidneys. International Journal of Nanomedicine, 14, 3995–4005. https://doi.org/10.2147/IJN.S200909
Ahamed, M., Ali, D., Alhadlaq, H. A., & Akhtar, M. J. (2013). Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2). Chemosphere, 93, 2514–2522. https://doi.org/10.1016/j.chemosphere.2013.09.047
Ahmad, J., Alhadlaq, H. A., Siddiqui, M. A., Saquib, Q., et al. (2015). Concentration-dependent induction of reactive oxygen species, cell cycle arrest and apoptosis in human liver cells after nickel nanoparticles exposure. Environmental Toxicology, 30, 137–148. https://doi.org/10.1002/tox.21879
Magaye, R. R., Yue, X., Zo, B., Shi, H., et al. (2014). acute toxicity of nickel nanoparticles in rats after intravenous injection. Int J Nanomedicine, 9, 1393–1402. https://doi.org/10.2147/IJN.S56212
Katsnelson, B. A., Minigaliyeva, I. A., Panov, V. G., Privalova, L. I., et al. (2015). Some patterns of metallic nanoparticles’ combined subchronic toxicity as exemplified by a combination of nickel and manganese oxide nanoparticles. Food Chem Toxicol, 86, 351–364. https://doi.org/10.1016/j.fct.2015.11.012
Yu, S., Liu, F., Wang, C., Zhang, J., et al. (2018). Role of oxidative stress in liver toxicity induced by nickel nanoparticles in rats. Molecular Medicine Reports, 17, 3133–3139. https://doi.org/10.3892/mmr.2017.8226
Razavipour, S. T., Behnammorshedi, M., Razavipour, R., et al. (2015). The toxic effect of nickel nanoparticles on oxidative stress and inflammatory markers. Biomedical Research, 26(2), 370–374.
Abudayyak, M., Guzel, E., & Özhan, G. (2017). Nickel oxide nanoparticles induce oxidative DNA damage and apoptosis in kidney cell line (NRK-52E). Biol Trace Elem Res, 178, 98–104. https://doi.org/10.1007/s12011-016-0892-z
Zhang, H., & Sun, S. (2015). NF-kB in inflammation and renal diseases. Cell Biosci, 5, 63–72. https://doi.org/10.1186/s13578-015-0056-4
S.Z. Abdulqadir,S.Z., and Aziz F.M.,(2019). Hepatotoxicity of nickel nanoparticles in rats Indian J Anim Res https://doi.org/10.18805/ijar. B-1100 Print ISSN:0367–6722 / Online ISSN:0976–0555
Garipi, A., Aksu, B., Akan, Z., Akakin, D., et al. (2011). Effect of extremely low frequency electromagnetic fields on growth rate and morphology of bacteria. International Journal of Radiation Biology, 87(12), 1155–1161.
Ali, F. M., Mohamed, W. S., & Mohamed, M. R. (2003). Effect of 50 Hz, 0.2 mT magnetic fields on RBC properties and heart functions of albino rats. Bioelectromagnetics, 24, 535–545. https://doi.org/10.1002/bem.10134
Wells, R. E., Denton, R., & Merrill, E. W. (1961). Measurement of viscosity of biologic fluids by cone plate viscometer. Journal of Laboratory and Clinical Medicine, 57, 646–656. https://doi.org/10.1248/cpb.34.4844
Patlolla, A. K., Barnes, C., Yedjou, C., Velma, V. R., Paul, B., & Tchounwou, P. B. (2009). Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley Rats. Environ Toxicol., 24(1), 66–73.
Ali, F.M., A, Gawish, A. M., Osman, M. B.S., Abdelbacki A. M., & El-Sharkawy., A.H. (2012). Control of Salmonella activity in rats by pulsed ELF magnetic field (In Vivo Study). Journal of International Dental and Medical Research, 5(2),129–135. http://www.ektodermaldisplazi.com/journal.htm
Orchard, G., & Nation, B. (2018). Histopathology. In Fundamentals of biomedical science (2nd Ed., pp. 520). Oxford.. https://global.oup.com/uk/orc/biosciences/biomed/orchard2e/
Dayani, M., Fathpour, H., & Naghsh, N. (2014). The effect of silver nanoparticles and thioacetamide on blood urea nitrogen and creatinine in male laboratory mice. International Journal of Biosciences, 4(1), 139–142. http://journal.skums.ac.ir/article-1-2203-fa.html
Griffitt, R. J., Luo, J., Gao, J., Bonzongo, J. C., & Barber, D. S. (2008). Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environmental Toxicology and Chemistry, 27(9), 1972–1978. https://doi.org/10.1897/08-002.1
Ispas, C., Andreescu, D., Patel, A., Goia, D. V., et al. (2009). Toxicity and developmental defects of different sizes and shape nickel nanoparticles in zebrafish. Environmental Science and Technology, 43, 6349–6356. August, 2009. https://doi.org/10.1021/es9010543
Carocci, A., Catalano, A., Lauria, G., et al. (2016). A review on mercury toxicity in food. In Food Toxicology; Debasis, B., Anand, S., Stohs, S.J., Eds.; CRC Press: Boca Raton, FL, USA, 2016; Chapter 16; pp. 315–326. https://doi.org/10.1201/9781315371443-17
Zambelli, B., & Ciurli, S. (2013). Nickel and human health. Met. Ions Life Sci, 13, 321–357. https://doi.org/10.3390/ijerph17030679
Seilkop, S. K., & Oller, A. R. (2003). Respiratory cancer risks associated with low-level nickel exposure: An integrated assessment based on animal, epidemiological, and mechanistic data. Regul. Toxicol. Pharm, 37, 173–190. https://doi.org/10.1016/S0273-2300(02)00029-6
Cao, L., Du, J., Ding, W., Jia, R., et al. (2016). Hepatoprotective and antioxidant effects of dietary Angelica sinensis extract against carbon tetrachloride-induced hepatic injury in Jian Carp (Cyprinus carpio var. Jian). Aquaculture Research, 47, 1852–1863. https://doi.org/10.1111/are.12643
Guo, H., Liu, H., Wu, H., Cui, H., et al. (2019). Nickel carcinogenesis mechanism: DNA damage. International Journal of Molecular Sciences, 20(19), 4690. https://doi.org/10.3390/ijms20194690
Yaqub, A., Anjum, K., Munir, A., Mukhtar, H., & Khan, W. (2018). Evaluation of acute toxicity and effects of sub-acute concentrations of copper oxide nanoparticles (CuO-NPs) on hematology, selected enzymes and histopathology of liver and kidney in Mus musculus. Indian Journal of Animal Research, 52(1), 92–98. https://doi.org/10.18805/ijar.v0iOF.8489
El Shahat, A. N., El Shennawy, H. M., & Abd El Megid, M. A. (2017). Studying the protective effect of gamma-irradiated basil (Ocimum basilicum L.) against methotrexate induced liver and renal toxicity in rats. Indian J Anim Res, 51(1), 135–140. https://doi.org/10.18805/ijar.9631
Morsy, G., & Elkon, N. (2014). Bioaccumulation of nickel nanopowder and evaluation of possible toxicity in male albino rats. Egyptian Journal of Zoology, 6, 275–299. https://doi.org/10.12816/0005519
Abdelhalim, M. A., Moussa, S. A., & Qaid, H. A. (2018). The protective role of quercetin and arginine on gold nanoparticles induced hepatotoxicity in rats. Int J Nano, 13, 2821–2825. https://doi.org/10.2147/IJN.S160995
Tammam, A.A., Khalaf, A.A., Zaki A.R., Khalifa M.M., Ibrahim M.A., et al.(2022).Hesperidin protects rats’ liver and kidney from oxidative damage and physiological disruption induced by nickel oxide nanoparticles. Frontiers in Psychology, 19:13,912625. https://doi.org/10.3389/fphys.2022.912625. PMID: 36338490; PMCID: PMC9626958.”
Kim, Y. S., Kim, J. S., Cho, H. S., Rha, D. S., et al. (2008). Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhalation Toxicology, 20(6), 575–583. https://doi.org/10.1080/08958370701874663
Zuckerman, J. E., Gale, A., Wu, P., et al. (2015). siRNA delivery to the glomerular mesangium using polycationic cyclodextrin nanoparticles containing siRNA. Nucleic Acid Therap, 25(2), 53–64. https://doi.org/10.1089/nat.2014.0505
Patri, A., Umbreit, T., Zheng, J., Nagashima, K., et al. (2009). Energy dispersive X-ray analysis of titanium dioxide nanoparticle distribution after intravenous and subcutaneous injection in mice.J. Appl. Toxicol, 29, 662–672. https://doi.org/10.1002/jat.1454
Fontana, L., Leso, V., Marinaccio, A., Cenacchi, G., et al. (2015). The effects of palladium nanoparticles on the renal function of female wistar rats. Nanotoxicology, 9(7), 843–851. https://doi.org/10.3109/17435390.2014.980759
Attia, A. (2014). Evaluation of the testicular alterations induced by silver nanoparticles in male mice: Biochemical, histological and ultrastructural studies. Res. J. Pharmac. Biol. And Chem, 5(4), 1558–1589.
Tiwari, R., Singh, R. D., Khan, H., et al. (2017). Oral subchronic exposure to silver nanoparticles causes renal damage through apoptotic impairment and necrotic cell death. Nanotoxicol, 11, 671–686. https://doi.org/10.1080/17435390.2017.1343874
Xu, X., Lai, Y., & Hua Z., (2019). Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci Rep, 39(1). 10.1042/BSR20180992
Aitken, R. J., & Roman, S. D. (2008). Antioxidant systems and oxidative stress in the testes. Oxidative Medicine and Cellular Longevity, 1(1), 15–24.
Dumala, N., Mangalampalli, B., Srinivas, S., Kamal, K., & Grover, P. (2018). Biochemical alterations induced by nickel oxide nanoparticles in female Wistar albino rats after acute oral exposure. Biomarkers, 23(1), 33–43. https://doi.org/10.1080/1354750X.2017.1360943
Kim, S., & Ryu, D. Y. (2013). Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. Journal of Applied Toxicology, 33, 78–89. https://doi.org/10.1002/jat.2792
Capasso, L., Camatini, M., & Gualtieri, M. (2014). Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells. Toxicology Letters, 226(1), 28–34. https://doi.org/10.1016/j.toxlet.2014.01.040
Arauz, J., Ramos-Tovar, E., & Muriel, P. (2016). Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Annals of Hepatology, 15(2), 160–173. https://doi.org/10.5604/16652681.1193701
Gustafson, H. H., Holt-Casper, D., Grainger, D. W., & Ghandehari, H. (2015). Nanoparticle uptake: The phagocyte problem. Nano Today, 10(4), 487–510. https://doi.org/10.1016/j.nantod.2015.06.006
Berrahal, A., Lasram, M., ElElj, N., Kerkeni, A., Gharb, I. N., & El-Fazaa, S. (2011). Effect of age-dependent exposure to lead on hepatotoxicity and nephrotoxicity in male rats. Environmental Toxicology, 26(1), 68. https://doi.org/10.1002/tox.20530
Ames, B. N., Shigenaga, M. K., & Hagen, T. M. (1993). Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National academy of Sciences of the United States of America, 90, 7915–7922. https://doi.org/10.1073/pnas.90.17.7915
Shigenaga, M. K., Gimeno, C. J., & Ames, B. N. (1989). Urinary 8-hydroxy-2′- deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proceedings of the National academy of Sciences of the United States of America, 86, 9697–9701. https://doi.org/10.1073/pnas.86.24.9697
Halliwell, B. (2000). Why and how should we measure oxidative damage in nutritional studies? How far could you come? American Journal of Clinical Nutrition, 72, 1082–1087. https://doi.org/10.1093/ajcn/72.5.1082
Kasai, H. (1997). Analysis of a form of oxidative DNA damage 8-hydroxy-2’-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutation Research, 387, 147–163. https://doi.org/10.1016/s1383-5742(97)00035-5
Rim, K. T., Song, S. W., & Kim, H. Y. (2013). Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: A literature review. Safety and health at work, 4(4), 177–186. https://doi.org/10.1016/j.shaw.2013.07.006
Steven, A., Lowe, J., Scott,I.,& Dam, Janov., I. Core pathology .3thed. Elsevier. China:442–443. ISBN: 978–0–7234–3444–3
Acknowledgements
The authors thank the Faculty of Science, Physics Department, Damanhour University, Egypt.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Sahar Abo-Neima, and Hadeer El-Sayed. Also, they carried out 5 ribonuclieotidase, 8-Hydroxy2′-Deoxyguanosine Experiment, biochemical analysis of tissue, histology, and Electron microscopy analysis of the liver. Sahar Abo-Neima studied dielectric relaxation for liver and kidney tissues, blood viscosity, blood film, and tissue conductivity. Noha Samak carried out the histopathology of the kidney. The first draft of the manuscript was written by Sahar Abo-Neima. Mostafa El-Sheekh supervised the work. The final version of the manuscript was revised and edited by Mostafa El-Sheekh. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This study was approved by the Scientific Induction Ethics Committee of Damanhour University by code number DMU-SCI-CSRE (22–10-02), and guidelines for the human care of animals were applied.
Consent to Participate
Not applicable.
Consent for Publication
All authors agree to publish this work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abo-Neima, S.E., El-Sheekh, M.M., Samak, N.M. et al. Nephrotoxicity, Hepatotoxicity, and Blood Viscoelasticity Induced by Nickel Nanoparticles in Albino Rats. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01421-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01421-0