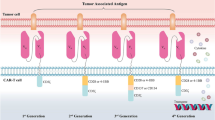
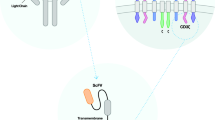
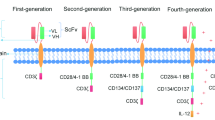
Abstract
Over the past decade, there has been continuous advancement in immunotherapy to improve human health. A promising technique that has emerged is chimeric antigen receptor (CAR)-T cell therapy, which presents an innovative approach to treating cancer. While CAR-T technology has shown success in treating blood cancers, it encounters challenges in addressing solid tumors. These challenges encompass the absence of dependable tumor-associated antigens, tumor heterogeneity, immunosuppressive tumor environments, and reduced T cell infiltration. To address these challenges, current research is focused on identifying dependable tumor-associated antigens and creating cost-effective, tumor microenvironment-specific CAR-T cells. These efforts aim to enhance the efficacy of CAR-T cell therapy for solid tumors. This review provides an overview of the development of CAR-T therapy for different types of tumors, encompassing both solid and hematological tumors. It outlines the challenges encountered in CAR-T cell therapy and proposes Approaches to address these obstacles.




Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
June, C. H., et al. (2018). CAR T cell immunotherapy for human cancer. Science, 359(6382), 1361–1365.
June, C. H., & Sadelain, M. (2018). Chimeric antigen receptor therapy. New England Journal of Medicine, 379(1), 64–73.
Sadelain, M., Rivière, I., & Riddell, S. (2017). Therapeutic T cell engineering. Nature, 545(7655), 423–431.
Sadelain, M., Brentjens, R., & Rivière, I. (2013). The basic principles of chimeric antigen receptor design. Cancer Discovery, 3(4), 388–398.
Kochenderfer, J. N., et al. (2012). B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood, The Journal of the American Society of Hematology, 119(12), 2709–2720.
Akbari, H., Mousazadeh, H., Akbarzadeh, A., et al. (2022). Co-loading of cisplatin and methotrexate in nanoparticle-based PCL-PEG system enhances lung cancer chemotherapy effects. Journal of Cluster Science, 33, 1751–1762.
Neelapu, S. S., et al. (2017). Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New England Journal of Medicine, 377(26), 2531–2544.
Abramson, J. S., et al. (2020). Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. The Lancet, 396(10254), 839–852.
Martin, T., et al. (2023). Ciltacabtagene autoleucel, an anti–B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. Journal of Clinical Oncology, 41(6), 1265.
Munshi, N. C., et al. (2021). Idecabtagene vicleucel in relapsed and refractory multiple myeloma. New England Journal of Medicine, 384(8), 705–716.
Lin, Y.-J., Mashouf, L. A., & Lim, M. (2022). CAR T cell therapy in primary brain tumors: Current investigations and the future. Frontiers in Immunology, 13, 817296.
Rafiq, S., Hackett, C. S., & Brentjens, R. J. (2020). Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nature reviews Clinical Oncology, 17(3), 147–167.
Newick, K., et al. (2017). CAR T cell therapy for solid tumors. Annual review of Medicine, 68, 139–152.
Keshavarz, A., et al. (2022). Recent findings on chimeric antigen receptor (CAR)-engineered immune cell therapy in solid tumors and hematological malignancies. Stem Cell Research & Therapy, 13(1), 1–22.
Patel, U., et al. (2022). CAR T cell therapy in solid tumors: A review of current clinical trials. EJHaem, 3, 24–31.
Chandran, S. S., & Klebanoff, C. A. (2019). T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance. Immunological Reviews, 290(1), 127–147.
Zhang, C., et al. (2017). Engineering car-t cells. Biomarker Research, 5(1), 1–6.
Lam, N., et al. (2020). Anti-BCMA chimeric antigen receptors with fully human heavy-chain-only antigen recognition domains. Nature Communications, 11(1), 283.
Nakajima, M., et al. (2019). Improved survival of chimeric antigen receptor-engineered T (CAR-T) and tumor-specific T cells caused by anti-programmed cell death protein 1 single-chain variable fragment-producing CAR-T cells. Cancer Science, 110(10), 3079–3088.
Alabanza, L., et al. (2017). Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Molecular Therapy, 25(11), 2452–2465.
Stornaiuolo, A., et al. (2021). Characterization and functional analysis of CD44v6. CAR T cells endowed with a new low-affinity nerve growth factor receptor-based spacer. Human Gene Therapy, 32(13–14), 744–760.
Morales, L., & Paramio, J. M. (2021). Cell therapies in bladder cancer management. International Journal of Molecular Sciences, 22(6), 2818.
Julamanee, J., et al. (2021). Composite CD79A/CD40 co-stimulatory endodomain enhances CD19CAR-T cell proliferation and survival. Molecular Therapy, 29(9), 2677–2690.
Dotti, G., et al. (2014). Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunological Reviews, 257(1), 107–126.
Sterner, R. C., & Sterner, R. M. (2021). CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer Journal, 11(4), 69.
Muller, Y. D., et al. (2021). The CD28-transmembrane domain mediates chimeric antigen receptor heterodimerization with CD28. Frontiers in Immunology, 12, 500.
Lanitis, E., Coukos, G., & Irving, M. (2020). All systems go: Converging synthetic biology and combinatorial treatment for CAR-T cell therapy. Current Opinion in Biotechnology, 65, 75–87.
Janeway, C. A. Jr, Travers, P., Walport, M., & Shlomchik, M. J. (2001). Antigen receptor structure and signaling pathways. In Immunobiology: The immune system in health and disease (5th ed.). Garland Science.
Hamieh, M., et al. (2023). Programming CAR T cell tumor recognition: Tuned antigen sensing and logic gating. Cancer Discovery, 13(4), 829–843.
Yan, T., Zhu, L., & Chen, J. (2023). Current advances and challenges in CAR T-Cell therapy for solid tumors: Tumor-associated antigens and the tumor microenvironment. Experimental Hematology & Oncology, 12(1), 14.
Brentjens, R. J., & Curran, K. J. (2012). Novel cellular therapies for leukemia: CAR-modified T cells targeted to the CD19 antigen. Hematology 2010, the American Society of Hematology Education Program Book, 1, 143–151.
Hiltensperger, M., & Krackhardt, A. M. (2023). Current and future concepts for the generation and application of genetically engineered CAR-T and TCR-T cells. Frontiers in Immunology, 14, 1121030.
Abbasi, S., Totmaj, M. A., Abbasi, M., Hajazimian, S., Goleij, P., Behroozi, J., Shademan, B., Isazadeh, A., & Baradaran, B. (2023). Chimeric antigen receptor T (CAR-T) cells: Novel cell therapy for hematological malignancies. Cancer Medicine, 12(7), 7844–7858.
Chmielewski, M., & Abken, H. (2015). TRUCKs: The fourth generation of CARs. Expert Opinion On Biological Therapy, 15(8), 1145–1154.
Parikh, R. H., & Lonial, S. (2023). Chimeric antigen receptor T-cell therapy in multiple myeloma: A comprehensive review of current data and implications for clinical practice. CA: A Cancer Journal for Clinicians, 73(3), 275–285.
Subklewe, M., von Bergwelt-Baildon, M., & Humpe, A. (2019). Chimeric antigen receptor T cells: A race to revolutionize cancer therapy. Transfusion Medicine and Hemotherapy, 46(1), 15–24.
Schepisi, G., et al. (2023). The new frontier of immunotherapy: Chimeric antigen receptor T (CAR-T) cell and macrophage (CAR-M) therapy against breast cancer. Cancers, 15(5), 1597.
Jan, M., et al. (2021). Reversible ON-and OFF-switch chimeric antigen receptors controlled by lenalidomide. Science Translational Medicine, 13(575), eabb6295.
Mellatyar, H., Talaei, S., & Nejati-Koshki, K. (2016). Targeting HSP90 gene expression with 17-DMAG nanoparticles in breast cancer cells. Asian Pacific Journal of Cancer Prevention, 17(5), 2453–2457.
Panahi, Y., Mohammadhosseini, M., Abadi, A. J., Akbarzadeh, A., & Mellatyar, H. (2016). An update on biomedical application of nanotechnology for Alzheimer’s disease diagnosis and therapy. Drug Research, 66(11), 580–586.
Norelli, M., et al. (2018). Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nature medicine, 24(6), 739–748.
Shimabukuro-Vornhagen, A., et al. (2018). Cytokine release syndrome. Journal for Immunotherapy of Cancer, 6(1), 1–14.
Santomasso, B., et al. (2019). The other side of CAR T-cell therapy: Cytokine release syndrome, neurologic toxicity, and financial burden. American Society of Clinical Oncology Educational Book, 39, 433–444.
Smith, L. T. (2017). Cytokine release syndrome: inpatient care for side effects of CAR T-cell therapy. Clinical Journal of Oncology Nursing, 21(2), 29–34.
Maude, S. L., et al. (2014). Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer Journal (Sudbury, Mass.), 20(2), 119.
Neelapu, S. S., et al. (2018). Toxicity management after chimeric antigen receptor T cell therapy: One size does not fit’ALL’. Nature Reviews Clinical Oncology, 15(4), 218–218.
Riegler, L. L., Jones, G. P., & Lee, D. W. (2019). Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Therapeutics and Clinical Risk Management, 15, 323–335.
Neelapu, S. S., et al. (2018). Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nature Reviews Clinical Oncology, 15(1), 47–62.
Locke, F. L., et al. (2017). Preliminary results of prophylactic tocilizumab after axicabtageneciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-Hodgkin lymphoma (NHL). Blood, 130, 1547.
Topp, M., et al. (2019). Earlier steroid use with axicabtagene ciloleucel (Axi-Cel) in patients with relapsed/refractory large B cell lymphoma. Blood, 134, 243.
Khan, A. N., et al. (2024). CAR-T cell therapy in hematological malignancies: Where are we now and where are we heading for? European Journal of Haematology, 112(1), 6–18.
Majzner, R. G., et al. (2020). Tuning the antigen density requirement for CAR T-cell activity. Cancer discovery, 10(5), 702–723.
Ramello, M. C., et al. (2019). An immunoproteomic approach to characterize the CAR interactome and signalosome. Science Signaling, 12(568), eaap9777.
Di Stasi, A., et al. (2011). Inducible apoptosis as a safety switch for adoptive cell therapy. New England Journal of Medicine, 365(18), 1673–1683.
Diaconu, I., et al. (2017). Inducible caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T cells. Molecular Therapy, 25(3), 580–592.
Santiago-Vicente, Y., de Jesús Castillejos-López, M., Carmona-Aparicio, L., Coballase-Urrutia, E., Velasco-Hidalgo, L., Niembro-Zúñiga, A. M., Zapata-Tarrés, M., & Torres-Espíndola, L. M. (2024). Immunotherapy for pediatric gliomas: CAR-T cells against B7H3: A review of the literature. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 23(4), 420–430.
Juillerat, A., et al. (2019). Modulation of chimeric antigen receptor surface expression by a small molecule switch. BMC biotechnology, 19, 1–9.
Gauthier, J., et al. (2020). Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood, The Journal of the American Society of Hematology, 135(19), 1650–1660.
Yu, H., et al. (2017). Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. American Journal of Hematology, 92(1), E11.
Taubmann, J., et al. (2024). Rescue therapy of antisynthetase syndrome with CD19-targeted CAR-T cells after failure of several B-cell depleting antibodies. Rheumatology, 63(1), e12–e14.
Hamieh, M., et al. (2019). CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature, 568(7750), 112–116.
Jackson, H. J., & Brentjens, R. J. (2015). Overcoming antigen escape with CAR T-cell therapy. Cancer Discovery, 5(12), 1238–1240.
Okada, M., Shimizu, K., & Fujii, S.-I. (2022). Identification of neoantigens in cancer cells as targets for immunotherapy. International Journal of Molecular Sciences, 23(5), 2594.
Hegde, M., et al. (2013). Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Molecular Therapy, 21(11), 2087–2101.
Zah, E., et al. (2016). T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunology Research, 4(6), 498–508.
Qin, H., et al. (2018). Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Molecular Therapy-Oncolytics, 11, 127–137.
Pan, J., et al. (2020). Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood, The Journal of the American Society of Hematology, 135(5), 387–391.
Goebeler, M.-E., & Bargou, R. C. (2020). T cell-engaging therapies—BiTEs and beyond. Nature Reviews Clinical Oncology, 17(7), 418–434.
Jen, E. Y., et al. (2019). FDA approval: Blinatumomab for patients with B-cell precursor acute lymphoblastic leukemia in morphologic remission with minimal residual disease. Clinical Cancer Research, 25(2), 473–477.
Borogovac, A., & Siddiqi, T. (2024). Transforming CLL management with immunotherapy: Investigating the potential of CAR T-cells and bispecific antibodies. In Seminars in hematology. WB Saunders.
Zhai, Y., Hong, J., Wang, J., Jiang, Y., Wu, W., Lv, Y., Guo, J., Tian, L., Sun, H., Li, Y., & Li, C. (2024). Comparison of blinatumomab and CAR T-cell therapy in relapsed/refractory acute lymphoblastic leukemia: A systematic review and meta-analysis. Expert Review of Hematology, 17(1–3), 67–76.
Subklewe, M. (2021). BiTEs better than CAR T cells. Blood Advances, 5(2), 607–612.
Chen, Y.-J., Abila, B., & Mostafa Kamel, Y. (2023). CAR-T: What is next? Cancers, 15(3), 663.
Choi, B. D., et al. (2019). CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nature Biotechnology, 37(9), 1049–1058.
Yang, J., et al. (2023). BCMA-targeting chimeric antigen receptor T-cell therapy for multiple myeloma. Cancer Letters, 553, 215949.
Krenciute, G., et al. (2016). 76. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells. Molecular Therapy, 24, S33.
Krenciute, G., et al. (2017). Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunology Research, 5(7), 571–581.
Zarrabi, K. K., et al. (2023). Bispecific PSMA antibodies and CAR-T in metastatic castration-resistant prostate cancer. Therapeutic Advances in Urology, 15, 17562872231182220.
Liu, X., Zhang, N., & Shi, H. (2017). Driving better and safer HER2-specific CARs for cancer therapy. Oncotarget, 8(37), 62730.
Cappell, K. M., & Kochenderfer, J. N. (2023). Long-term outcomes following CAR T cell therapy: What we know so far. Nature Reviews Clinical Oncology, 20(6), 359–371.
Yang, J., et al. (2023). Advancing CAR T cell therapy through the use of multidimensional omics data. Nature Reviews Clinical Oncology, 20(4), 211–228.
Bhat, A. A., et al. (2021). Cytokine-chemokine network driven metastasis in esophageal cancer; promising avenue for targeted therapy. Molecular Cancer, 20(1), 1–20.
Nisar, S., et al. (2021). Chemokine-cytokine networks in the head and neck tumor microenvironment. International Journal of Molecular Sciences, 22(9), 4584.
Ager, A. (2017). High endothelial venules and other blood vessels: Critical regulators of lymphoid organ development and function. Frontiers in Immunology, 8, 45.
Yang, J., Yan, J., & Liu, B. (2018). Targeting VEGF/VEGFR to modulate antitumor immunity. Frontiers in Immunology, 9, 978.
Zhang, P., Zhang, G., & Wan, X. (2023). Challenges and new technologies in adoptive cell therapy. Journal of Hematology & Oncology, 16(1), 97.
Zhang, B.-L., et al. (2016). Hurdles of CAR-T cell-based cancer immunotherapy directed against solid tumors. Science China Life Sciences, 59, 340–348.
Caruana, I., et al. (2015). Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nature Medicine, 21(5), 524–529.
Can, C. A. R. T. C., & Growth, I. T. (2014). Targeting fibroblast activation protein in tumor stroma with. Cancer, 2(2), 154.
Moon, E. K., et al. (2018). Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology, 7(3), e1395997.
Adachi, K., et al. (2018). IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nature Biotechnology, 36(4), 346–351.
Tchou, J., et al. (2017). Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunology Research, 5(12), 1152–1161.
Nellan, A., et al. (2018). Durable regression of medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. Journal for Immunotherapy of Cancer, 6(1), 1–14.
Klampatsa, A., et al. (2017). Intracavitary ‘T4 immunotherapy’of malignant mesothelioma using pan-ErbB re-targeted CAR T-cells. Cancer Letters, 393, 52–59.
Katz, S., et al. (2016). Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Therapy, 23(5), 142–148.
Zhu, W. M., & Middleton, M. R. (2023). Combination therapies for the optimisation of bispecific T-cell engagers in cancer treatment. Immunotherapy Advances, 3(1), ltad013.
ElSamadisy, O., et al. (2024). Safe, efficient, and comfortable reinforcement-learning-based car-following for AVs with an analytic safety guarantee and dynamic target speed. Transportation Research Record, 2678(1), 643–661.
Fisher, J., et al. (2017). Avoidance of on-target off-tumor activation using a co-stimulation-only chimeric antigen receptor. Molecular Therapy, 25(5), 1234–1247.
Schneider, M., et al. (2015). CD38 is expressed on inflammatory cells of the intestine and promotes intestinal inflammation. PLoS ONE, 10(5), e0126007.
Mizuguchi, M., et al. (1995). Neuronal localization of CD38 antigen in the human brain. Brain Research, 697(1–2), 235–240.
Dwivedi, S., Rendón-Huerta, E. P., Ortiz-Navarrete, V., & Montaño, L. F. (2021). CD38 and regulation of the immune response cells in cancer. Journal of Oncology, 2021, 6630295.
Steentoft, C., et al. (2018). Glycan-directed CAR-T cells. Glycobiology, 28(9), 656–669.
Pinto, S. N., & Krenciute, G. (2024). The mechanisms of altered blood–brain barrier permeability in CD19 CAR T–cell recipients. International Journal of Molecular Sciences, 25(1), 644.
Suarez, E. R., et al. (2016). Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget, 7(23), 34341.
Zhu, X., et al. (2017). CAR-T cell therapy in ovarian cancer: From the bench to the bedside. Oncotarget, 8(38), 64607.
Morgan, R. A., et al. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy, 18(4), 843–851.
Daei Sorkhabi, A., et al. (2023). The current landscape of CAR T-cell therapy for solid tumors: Mechanisms, research progress, challenges, and counterstrategies. Frontiers in Immunology, 14, 1113882.
Moradi, V., Omidkhoda, A., & Ahmadbeigi, N. (2023). The paths and challenges of “off-the-shelf” CAR-T cell therapy: An overview of clinical trials. Biomedicine & Pharmacotherapy, 169, 115888.
Kringel, R., Lamszus, K., & Mohme, M. (2023). Chimeric antigen receptor T cells in glioblastoma—Current concepts and promising future. Cells, 12(13), 1770.
Park, S., et al. (2017). Micromolar affinity CAR T cells to ICAM-1 achieves rapid tumor elimination while avoiding systemic toxicity. Scientific Reports, 7(1), 14366.
Oluwole, O. O., et al. (2021). Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. British Journal of Haematology, 194(4), 690–700.
Fry, T. J., et al. (2018). CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nature Medicine, 24(1), 20–28.
Fry, T. J., et al. (2018). CD22-CAR T cells induce remissions in CD19-CAR naïve and resistant B-ALL. Nature Medicine, 24(1), 20.
Jia, Q., et al. (2022). Heterogeneity of the tumor immune microenvironment and its clinical relevance. Experimental Hematology & Oncology, 11(1), 24.
Liu, G., et al. (2021). Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cellular & Molecular Immunology, 18(5), 1085–1095.
Lin, H.-W., Liu, J.-Y., & Luo, W.-X. (2023). Advances in CAR-T combination therapy for solid tumors. China Biotechnology, 42(12), 37–51.
Obiorah, I., & Courville, E. L. (2023). Diagnostic flow cytometry in the era of targeted therapies: Lessons from therapeutic monoclonal antibodies and chimeric antigen receptor T-cell adoptive immunotherapy. Surgical Pathology Clinics, 16(2), 423–431.
Holstein, S. A., Grant, S. J., & Wildes, T. M. (2023). Chimeric antigen receptor T-cell and bispecific antibody therapy in multiple myeloma: Moving into the future. Journal of Clinical Oncology, 41(27), 4416–4429.
Aparicio, C., et al. (2021). Cell therapy for colorectal cancer: The promise of chimeric antigen receptor (CAR)-T cells. International Journal of Molecular Sciences, 22(21), 11781.
Pearlman, A. H., et al. (2021). Targeting public neoantigens for cancer immunotherapy. Nature Cancer, 2(5), 487–497.
Lang, F., et al. (2022). Identification of neoantigens for individualized therapeutic cancer vaccines. Nature reviews Drug discovery, 21(4), 261–282.
Marques-Piubelli, M. L., Kim, D. H., Medeiros, L. J., Lu, W., Khan, K., Gomez-Bolanos, L. I., Rodriguez, S., Parra, E. R., Ok, C. Y., Aradhya, A., & Solis, L. M. (2023). CD30 expression is frequently decreased in relapsed classic Hodgkin lymphoma after anti-CD30 CAR T-cell therapy. Histopathology, 83(1), 143–148.
Blass, E., & Ott, P. A. (2021). Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nature Reviews Clinical Oncology, 18(4), 215–229.
Zhang, Q., et al. (2022). Neoantigens in precision cancer immunotherapy: From identification to clinical applications. Chinese Medical Journal, 135(11), 1285–1298.
Gao, J., et al. (2021). Complete rejection of large established breast cancer by local immunochemotherapy with T cell activation against neoantigens. Cancer Immunology, Immunotherapy, 70, 3291–3302.
Ross, S. L., et al. (2017). Bispecific T cell engager (BiTE®) antibody constructs can mediate bystander tumor cell killing. PLoS ONE, 12(8), e0183390.
Cho, J. H., Collins, J. J., & Wong, W. W. (2018). Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell, 173(6), 1426-1438.e11.
Ventin, M., et al. (2023). B7-H3-targeted CAR T cell activity is enhanced by radiotherapy in solid cancers. Frontiers in Oncology, 13, 1193963.
Bach, P. B., Giralt, S. A., & Saltz, L. B. (2017). FDA approval of tisagenlecleucel: Promise and complexities of a $475 000 cancer drug. JAMA, 318(19), 1861–1862.
Acknowledgements
The authors would like to thank the Department of Medical Nanotechnology, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, for financially supporting this project (grant no.: 64379).
Funding
This study was funded by the Department of Medical Nanotechnology, Faculty of Advanced Medical Sciences, and Tabriz University of Medical Sciences (grant no.: 64379).
Author information
Authors and Affiliations
Contributions
"AA conceptualized research; ANA, and ST supervised research; HM performed research and analyzed data, and prepared figures.All authors reviewed the manuscript."
Corresponding author
Ethics declarations
Ethical Approval
The ethical approval for this paper was obtained from research ethics committee of Tabriz University of Medical Sciences)IR.TBZMED.VCR.REC.1397.487(.
Ethics Approval and Consent to Participate
N/A.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mellatyar, H., Sattari, S., Asl, A.N. et al. Recent Advances and Challenges in Cancer Treatment with Car T Cell Therapy: A Novel Anti-cancer Strategy. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01389-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01389-x