Abstract
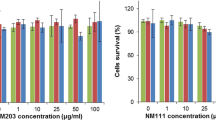
Silica nanoparticles (SiNPs) demonstrate exceptional properties that vary depending on their intended applications. It is imperative to comprehend the toxic effects associated with these nanoparticles, which have gained wider use due to diverse synthesis methods and application areas. The sol-gel method yields SiNPs with a radius ranging from 4 to 7 nm. The characterization of the synthesized nanoparticles involved capturing transmission electron microscopy images (TEM), employing Fourier transform infrared spectroscopy (FTIR), and conducting differential scanning calorimetry (DSC). The evaluation of their toxicity and cellular uptake was performed on human vein endothelial (HUVEC) cells. The impact on the model organism Cearnohabditis elegans (C. elegans) and bacterial growth was also investigated. The zeta sizer measurements confirmed the average size distribution of 7 nm, while also revealing a strong surface charge of the particles. The presence of Si–O–Si junctions was indicated by the peak observed at 1077 cm−1 on the FTIR spectrum of the SiNPs. The thermogram obtained from the DSC measurement, conducted between 200 and 500 °C, displayed a similar curve to polymer matrices containing SiNPs. The cytotoxicity experiments were focused on investigating the effects of low concentrations of SiNPs. Even at 10 μg/mL, 50 μg/mL, and 100 μg/mL concentrations, increasing concentrations resulted in a decrease in cell proliferation. Antibacterial effects were confirmed by a real-time cell analyzer (xCELLigence) for 24 h. At a concentration of 10 μg/mL, there were no observable effects on lifespan, reproduction, and body bending frequency; at a 50 μg/mL, the nanoparticles accumulated in the nematode body affected the body bending frequency.






Similar content being viewed by others
Data Availability
The datasets used and materials presented in this study are available upon reasonable request, and the author is open to sharing the data for further scientific inquiry and collaboration.
References
Wang, G., Yau, S.-T., Mantey, K., & Nayfeh, M. H. (2008). Fluorescent Si nanoparticle-based electrode for sensing biomedical substances. Optics Communication, 281(7), 1765–1770.
Nayfeh, M. H., & Mitas, L. (2008). Silicon nanoparticles: new photonic and electronic material at the transition between solid and molecule. Nanosilicon, 1–78.
Lu, J., Liong, M., Li, Z., Zink, J. I., & Tamanoi, F. (2010). Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small, 6(16), 1794–1805.
Croissant, J. G., Butler, K. S., Zink, J. I., & Brinker, C. J. (2020). Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nature Reviews Materials, 5(12), 886–909.
Sayes, C. M., Reed, K. L., Glover, K. P., Swain, K. A., Ostraat, M. L., Donner, E. M., & Warheit, D. B. (2010). Changing the dose metric for inhalation toxicity studies: Short-term study in rats with engineered aerosolized amorphous silica nanoparticles. Inhalation Toxicology, 22(4), 348–354.
Li, Y., Sun, L., Jin, M., Du, Z., Liu, X., Guo, C., Li, Y., Huang, P., Sun, Z. (2011). Size-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma HepG2 cells. Toxicology In Vitro, 25(7), 1343–1352.
Napierska, D., Thomassen, L. C., Rabolli, V., Lison, D., Gonzalez, L., Kirsch‐Volders, M., Martens, J. A., & Hoet, P. H. (2009). Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small, 5(7), 846–853.
Yazdimamaghani, M., Moos, P. J., Dobrovolskaia, M. A., & Ghandehari, H. (2019). Genotoxicity of amorphous silica nanoparticles: Status and prospects. Nanomedicine: Nanotechnology, Biology and Medicine, 16, 106–125.
Chen, L., Liu, J., Zhang, Y., Zhang, G., Kang, Y., Chen, A., Feng, X., & Shao, L. (2018). The toxicity of silica nanoparticles to the immune system. Nanomedicine, 13(15), 1939–1962.
Petushkov, A., Ndiege, N., Salem, A. K., & Larsen, S. C. (2010). Toxicity of silica nanomaterials: zeolites, mesoporous silica, and amorphous silica nanoparticles. In Advances in Molecular Toxicology, 4, 223–266. Elsevier.
Freese, C., Schreiner, D., Anspach, L., Bantz, C., Maskos, M., Unger, R. E., & Kirkpatrick, C. J. (2014). In vitro investigation of silica nanoparticle uptake into human endothelial cells under physiological cyclic stretch. Particle and fibre toxicology, 11(1), 1–12.
Mathelié-Guinlet, M., Béven, L., Moroté, F., Moynet, D., Grauby-Heywang, C., Gammoudi, I., & Cohen-Bouhacina, T. (2017). Probing the threshold of membrane damage and cytotoxicity effects induced by silica nanoparticles in Escherichia coli bacteria. Advances in Colloid and Interface Science, 245, 81–91.
Liu, J. Y., & Sayes, C. M. (2022). A toxicological profile of silica nanoparticles. Toxicology Research, 11(4), 565–582.
Kosyan, D., Yausheva, E., Vasilchenko, A., Vasilchenko, A., & Miroshnikov, S. (2017). Toxicity of SiO2, TiO2 and CeO2 nanoparticles evaluated using the bioluminescence assay. GEOMATE Journal, 13(40), 66–73.
Gamze, A., & Şifa, T. (2020). The investigation of toxic, genotoxic and cytotoxic effects of various nanoparticles in Allium cepa and Caenorhabditis elegans test systems. World Journal of Advanced Research and Reviews, 5(1), 016–035.
Wang, Q., Zhu, Y., Song, B., Fu, R., & Zhou, Y. (2022). The In vivo toxicity assessments of water-dispersed fluorescent silicon nanoparticles in Caenorhabditis elegans. International Journal of Environmental Research and Public Health, 19(7), 4101.
Que, D. E., Hou, W.-C., Ang, M. B. M. Y., & Lin, C.-C. (2020). Toxic effects of hydroxyl-and amine-functionalized silica nanoparticles (SiO2 and NH2-SiO2 NPs) on Caenorhabditis elegans. Aerosol and Air Quality Research, 20(9), 1987–2020.
Acosta, C., Barat, J. M., Martínez-Máñez, R., et al. (2018). Toxicological assessment of mesoporous silica particles in the nematode Caenorhabditis elegans. Environmental Research, 166, 61–70.
Liljenström, C., Lazarevic, D., & Finnveden, G. (2013). Silicon-based nanomaterials in a life-cycle perspective, including a case study on self-cleaning coatings. KTH Royal Institute of Technology.
Bitar, A., Ahmad, N. M., Fessi, H., & Elaissari, A. (2012). Silica-based nanoparticles for biomedical applications. Drug Discovery Today, 17(19-20), 1147–1154.
Dinç, B., Ünlü, A., & Bektaş, M. (2020). Characterization of short-length multi-walled carbon nanotubes and cytotoxicity on MDA-MB-231 and HUVEC cell lines. Carbon Letters, 30(2), 143–153.
Zhang, M., Yang, M., Okazaki, T., & Yudasaka, M. (2018). Quantification of carbon nanotubes taken up by macrophage cells using optical absorption method. e-Journal of Surface Science and Nanotechnology, 16, 93–96.
Laaksonen, T., Santos, H., Vihola, H., H., Salonen, J., Riikonen, J., Heikkilä, T., Peltonen, L., Kumar, N., Murzin, D. Y., Lehto, V-P., & Hirvonen, J. (2007). Failure of MTT as a toxicity testing agent for mesoporous silicon microparticles. Chemical Research in Toxicology, 20(12), 1913–1918.
Balakrishnan, V., Ab Wab, H. A., Razak, K. A., & Shamsuddin, S. (2013). In vitro evaluation of cytotoxicity of colloidal amorphous silica nanoparticles designed for drug delivery on human cell lines. Journal of Nanomaterials, 2013, 4–4.
Gade, A., Bonde, P., Ingle, A., Marcato, P., Duran, N., & Rai, M. (2008). Exploitation of Aspergillus niger for synthesis of silver nanoparticles. Journal of Biobased Materials and Bioenergy, 2(3), 243–247.
Ünlü, A., Meran, M., Dinc, B., Karatepe, N., Bektaş, M., & Güner, F. S. (2018). Cytotoxicity of doxrubicin loaded single-walled carbon nanotubes. Molecular Biology Reports, 45, 523–531.
Dinc, B., & Sen, E. (2022). Toxicity of short multi-walled carbon nanotubes in Caenorhabditis elegans. Fullerenes, Nanotubes, and Carbon Nanostructures, 30(6), 646–656.
Larsson, M., Hill, A., & Duffy, J. (2012). Suspension stability; why particle size, zeta potential and rheology are important. Annual Transactions of the Nordic Rheology Society, 20(6).
Eid, M. M. (2022). Characterization of nanoparticles by FTIR and FTIR-microscopy. In Handbook of Consumer Nanoproducts (pp. 1–30). Springer.
Borrajo, J. P., Liste, S., Serra, J., González, P., Chiussi, S., León, B., Amor, M. P., Ylänen, H. O., & Hupa, M. (2004). Influence of the network modifier content on the bioactivity of silicate glasses. Key Engineering Materials, 254, 23–26.
Ono, H., Ikarashi, T., Miura, Y., Hasegawa, E., Ando, K., & Kitano, T. (1999). Bonding configurations of nitrogen absorption peak at 960 cm− 1 in silicon oxynitride films. Applied Physics Letters, 74(2), 203–205.
Vallhov, H., Gabrielsson, S., Strømme, M., Scheynius, A., & Garcia-Bennett, A. E. (2007). Mesoporous silica particles induce size dependent effects on human dendritic cells. Nano Letters, 7(12), 3576–3582. https://doi.org/10.1021/nl0714785
Leclerc, L., Rima, W., Boudard, D., Pourchez, J., Forest, V., Bin, V., Movat, P., Perriat, P., Tillement, O., Grosseau, P., Bernache-Assollant, D., & Cottier, M. (2012). Size of submicrometric and nanometric particles affect cellular uptake and biological activity of macrophages in vitro. Inhalation Toxicology, 24(9), 580–588.
Andreeva, E., Rudimov, E., Gornostaeva, A., Beklemyshev, V. I., Makhonin, I. I., Maugeri, U. O. G., & Buravkova, L. B. (2013). In vitro study of interactions between silicon-containing nanoparticles and human peripheral blood leukocytes. Bulletin of Experimental Biology and Medicine, 155, 396–398.
Huang, X., Li, L., Liu, T., Hao, N., Liu, H., Chen, D., & Tang, F. (2011). The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano, 5(7), 5390–5399.
Selvarajan, V., Obuobi, S., & Ee, P. L. R. (2020). Silica nanoparticles—A versatile tool for the treatment of bacterial infections. Frontiers in Chemistry, 8, 602.
Smirnov, N., Kudryashov, S., Nastulyavichus, A., Rudenko, A. A., Saraeva, I. N., Tolordava, E. R., Gonchukov, S. A., Ramanova , Y. M., Ionin, A. A., & Zayarny, D. A. (2018). Antibacterial properties of silicon nanoparticles. Laser Physics Letters, 15(10), 105602.
Hetrick, E. M., Shin, J. H., Paul, H. S., & Schoenfisch, M. H. (2009). Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials, 30(14), 2782–2789.
Jabir, M. S., Nayef, U. M., Jawad, K. H., Taqi, Z. J., & Ahmed, N. R. (2018). Porous silicon nanoparticles prepared via an improved method: a developing strategy for a successful antimicrobial agent against Escherichia coli and Staphylococcus aureus (p. 012077). IOP Publishing.
Bernardos, A., Piacenza, E., Sancenón, F., M., Maleki, A., Turner, R. J., & Martínez‐Máñez, R.(2019). Mesoporous silica-based materials with bactericidal properties. Small, 15(24), 1900669.
Zhou, Y., Zhang, Y., Zhong, Y., Fu, R., Wu, S., Wang, Q., Wang, H., Su, Y., Zhang, H., He, Y. (2018). The in vivo targeted molecular imaging of fluorescent silicon nanoparticles in Caenorhabditis elegans. Nano Research, 11, 2336–2346.
Huang, C. W., Li, S. W., & Liao, V. H. C. (2019). Long-term sediment exposure to ZnO nanoparticles induces oxidative stress in Caenorhabditis elegans. Environmental Science: Nano, 6(8), 2602–2614.
Croissant, J. G., Butler, K. S., Zink, J. I., & Brinker, C. J. (2020). Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nature Reviews Materials, 5(12), 886–909.
Dong, X., Wu, Z., Li, X., Xiao, L., Yang, M., Li, Y., & Sun, Z. (2020). The size-dependent cytotoxicity of amorphous silica nanoparticles: a systematic review of in vitro studies. International Journal of Nanomedicine, 9089–9113.
Bosch, A., Bott, J., Warfving, N., & Nolde, J. (2023). Investigation on the skin penetration of synthetic amorphous silica (SAS) used in Cosmetic Products. Toxicology Letters.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
While ethical approval was not mandated for C. elegans research, all experiments were conducted in adherence to ethical principles, ensuring proper care and handling of the organisms, minimizing distress, justifying experimental necessity, and maintaining transparency in reporting methods and results.
Competing Interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dinc, B. Toxicity of Sol-Gel Synthesized Silica Nanoparticles: Insights from Cellular and Model Organism Studies. BioNanoSci. 13, 1922–1932 (2023). https://doi.org/10.1007/s12668-023-01186-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01186-y