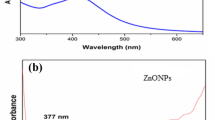
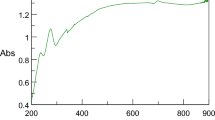
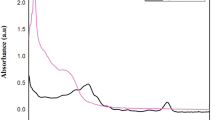
Abstract
Plant-derived compounds with anti-cancer effects are known as new candidates for treatment. Silymarin is one of these effective compounds; however, the low solubility of silymarin in aqueous systems can be one of the possible obstacles. Nanotechnology has been used to increase the solubility of poorly water-soluble drugs. In this study, silymarin was delivered with silver nanoparticles (AgNPs) to breast cancer cells. Nanoparticles were synthesized by the green method using gallic acid. Silymarin loading into nanoparticles was confirmed by FTIR. The toxicity of AgNPs, silymarin, and AgNPs/silymarin on two different breast cancer lines (MCF7 and MDA-MB-231) and normal HDF cells was investigated. According to results, AgNPs/silymarin exhibited a greater lethal effect on cancer lines compared to single treatment and much less lethal effect on the HDF cell. Inhibition of colony formation, migration and increased apoptosis were found to be effective on the treatment with AgNPs/silymarin compared to single treatments. The highest decrease in the expression of genes involved in metastasis, KRAS, MMP2/9, and TPM3 was observed. The use of AgNPs to deliver silymarin to cancer cells creates different inhibitory mechanisms against cancer cells, which highlights the use of AgNPs as drug delivery systems in cancer.










Similar content being viewed by others
Data Availability
The authors declare that all generated and analyzed data are included in this article.
References
Boisselier, E., & Astruc, D. (2009). Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chemical Society Reviews, 38(6), 1759–1782.
Iyer, A. K., He, J., & Amiji, M. M. (2012). Image-guided nanosystems for targeted delivery in cancer therapy. Current Medicinal Chemistry, 19(19), 3230–3240.
Sargazi, S., et al. (2022). Application of green gold nanoparticles in cancer therapy and diagnosis. Nanomaterials, 12(7), 1102.
Holgado, M. A., Martin-Banderas, L., Alvarez-Fuentes, J., Fernandez-Arevalo, M., & Arias, J. L. (2012). Drug targeting to cancer by nanoparticles surface functionalized with special biomolecules. Current Medicinal Chemistry, 19(19), 3188–3195.
Khan, M. I., et al. (2022). Recent progress in nanostructured smart drug delivery systems for cancer therapy: A review. ACS Applied Bio Materials, 5(3), 971–1012.
Pradhan, S., & Girish, C. (2006). Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian Journal of Medical Research, 124(5), 491.
Manna, S. K., Mukhopadhyay, A., Van, N. T., & Aggarwal, B. B. (1999). Silymarin suppresses TNF-induced activation of NF-κB, c-Jun N-terminal kinase, and apoptosis. The Journal of Immunology, 163(12), 6800–6809.
Morazzoni, P., Montalbetti, A., Malandrino, S., & Pifferi, G. (1993). Comparative pharmacokinetics of silipide and silymarin in rats. European Journal of Drug Metabolism and Pharmacokinetics, 18(3), 289–297.
Morais, M., Teixeira, A. L., Dias, F., Machado, V., Medeiros, R., & Prior, J. A. (2020). Cytotoxic effect of silver nanoparticles synthesized by green methods in cancer. Journal of Medicinal Chemistry, 63(23), 14308–14335.
Gomes, H. I., Martins, C. S., & Prior, J. A. (2021). Silver nanoparticles as carriers of anticancer drugs for efficient target treatment of cancer cells. Nanomaterials, 11(4), 964.
Barabadi, H., Ovais, M., Shinwari, Z. K., & Saravanan, M. (2017). Anti-cancer green bionanomaterials: Present status and future prospects. Green Chemistry Letters and Reviews, 10(4), 285–314.
Das, R. K., et al. (2017). Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnology for Environmental Engineering, 2(1), 1–21.
Cheng, N., et al. (2019). Weighted gene co-expression network analysis reveals specific modules and hub genes related to neuropathic pain in dorsal root ganglions. Bioscience Reports, 39(11), BSR20191511.
Puttaraju, N. G., Venkateshaiah, S. U., Dharmesh, S. M., Urs, S. M. N., & Somasundaram, R. (2006). Antioxidant activity of indigenous edible mushrooms. Journal of Agricultural and Food Chemistry, 54(26), 9764–9772.
Chia, Y.-C., Rajbanshi, R., Calhoun, C., & Chiu, R. H. (2010). Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules, 15(11), 8377–8389.
Kim, Y.-J. (2007). Antimelanogenic and antioxidant properties of gallic acid. Biological and Pharmaceutical Bulletin, 30(6), 1052–1055.
Kratz, J. M., et al. (2008). Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate. Memórias do Instituto Oswaldo Cruz, 103(5), 437–442.
Alam, B., et al. (2013). Antioxidant, analgesic and anti-inflammatory activities of the methanolic extract of Piper betle leaves. Avicenna Journal of Phytomedicine, 3(2), 112.
Kubo, I., Fujita, K. I., Nihei, K. I., & Masuoka, N. (2003). Non-antibiotic antibacterial activity of dodecyl gallate. Bioorganic & Medicinal Chemistry, 11(4), 573–580.
Kubo, I., Xiao, P., & Fujita, K. I. (2001). Antifungal activity of octyl gallate: Structural criteria and mode of action. Bioorganic & Medicinal Chemistry Letters, 11(3), 347–350.
Graham, L. J., et al. (2014). Current approaches and challenges in monitoring treatment responses in breast cancer. Journal of Cancer, 5(1), 58.
McAnena, P., Lowery, A., & Kerin, M. (2018). Role of micro-RNAs in breast cancer surgery. British Journal of Surgery, 105(2), e19–e30.
Liu, Y., Hussain, M., Wang, X., Liu, B., & Yuan, Y. (2020). Anti-tumor activity of verbascoside-loaded noble metal nanoparticles. In Nanoparticle Drug Delivery Systems for Cancer Treatment (pp. 271–294). Jenny Stanford Publishing.
Jaggi, A. S., & Singh, N. (2016). Silymarin and its role in chronic diseases. Drug Discovery from Mother Nature, 25–44.
Amirsaadat, S., Jafari-Gharabaghlou, D., Alijani, S., Mousazadeh, H., Dadashpour, M., & Zarghami, N. (2021). Metformin and Silibinin co-loaded PLGA-PEG nanoparticles for effective combination therapy against human breast cancer cells. Journal of Drug Delivery Science and Technology, 61, 102107.
Xie, Y., Zhang, D., Zhang, J., & Yuan, J. (2019). Metabolism, transport and drug–drug interactions of silymarin. Molecules, 24(20), 3693.
Park, J., Cha, S.-H., Cho, S., & Park, Y. (2016). Green synthesis of gold and silver nanoparticles using gallic acid: Catalytic activity and conversion yield toward the 4-nitrophenol reduction reaction. Journal of Nanoparticle Research, 18, 1–13.
Zhang, T., et al. (2019). Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncology Reports, 41(3), 1779–1788.
Jiang, Y., Pei, J., Zheng, Y., Miao, Y. J., Duan, B. Z., & Huang, L. F. (2021). Gallic acid: A potential anti-cancer agent. Chinese Journal of Integrative Medicine, 1–11.
Shakeran, Z., Keyhanfar, M., Varshosaz, J., & Sutherland, D. S. (2021). Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Materials Science and Engineering: C, 118, 111526.
Dadashpour, M., et al. (2018). Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Materials Science and Engineering: C, 92, 902–912.
Anand, K., et al. (2016). Biosynthesis of palladium nanoparticles by using Moringa oleifera flower extract and their catalytic and biological properties. Journal of Photochemistry and Photobiology B: Biology, 165, 87–95.
Kummara, S., Patil, M. B., & Uriah, T. (2016). Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles–A comparative study. Biomedicine & Pharmacotherapy, 84, 10–21.
Delmas, D., Xiao, J., Vejux, A., & Aires, V. (2020). Silymarin and cancer: A dual strategy in both in chemoprevention and chemosensitivity. Molecules, 25(9), 2009.
Atawia, R. T., Tadros, M. G., Khalifa, A. E., Mosli, H. A., & Abdel-Naim, A. B. (2013). Role of the phytoestrogenic, pro-apoptotic and anti-oxidative properties of silymarin in inhibiting experimental benign prostatic hyperplasia in rats. Toxicology letters, 219(2), 160–169.
Koltai, T., & Fliegel, L. (2022). "Role of silymarin in cancer treatment: Facts, hypotheses, and questions," Journal of Evidence-Based. Integrative Medicine, 27, 2515690X211068826.
Ramteke, L., Gawali, P., Jadhav, B., & Chopade, B. (2020). Comparative study on antibacterial activity of metal ions, monometallic and alloy noble metal nanoparticles against nosocomial pathogens. BioNanoScience, 10(4), 1018–1036.
Singh, R. P., & Ramarao, P. (2012). Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicology Letters, 213(2), 249–259.
Patra, S., Mukherjee, S., Barui, A. K., Ganguly, A., Sreedhar, B., & Patra, C. R. (2015). Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Materials Science and Engineering: C, 53, 298–309.
Javan, E. S., Lotfi, F., Jafari-Gharabaghlou, D., Mousazadeh, H., Dadashpour, M., & Zarghami, N. (2022). Development of a magnetic nanostructure for co-delivery of metformin and silibinin on growth of lung cancer cells: Possible action through leptin gene and its receptor regulation. Asian Pacific Journal of Cancer Prevention: APJCP, 23(2), 519.
Gurunathan, S., Han, J. W., Eppakayala, V., Jeyaraj, M., & Kim, J.-H. (2013). Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. BioMed Research International, 2013, 10. https://doi.org/10.1155/2013/535796
Jeyaraj, M., et al. (2013). An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids and Surfaces B: Biointerfaces, 102, 708–717.
Zada, S., et al. (2018). Biogenic synthesis of silver nanoparticles using extracts of Leptolyngbya JSC-1 that induce apoptosis in HeLa cell line and exterminate pathogenic bacteria. Artificial Cells, Nanomedicine, and Biotechnology, 46(sup3), 471–480.
Khalili, H., Sadat Shandiz, S. A., & Baghbani-Arani, F. (2017). Anticancer properties of phyto-synthesized silver nanoparticles from medicinal plant Artemisia tschernieviana Besser aerial parts extract toward HT29 human colon adenocarcinoma cells. Journal of Cluster Science, 28, 1617–1636.
Fan, L., Ma, Y., Liu, Y., Zheng, D., & Huang, G. (2014). Silymarin induces cell cycle arrest and apoptosis in ovarian cancer cells. European Journal of Pharmacology, 743, 79–88.
Kim, S. H., et al. (2019). Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells. Oncology Reports, 42(5), 1904–1914.
Hosseinabadi, T., et al. (2019). Silymarin antiproliferative and apoptotic effects: Insights into its clinical impact in various types of cancer. Phytotherapy Research, 33(11), 2849–2861.
Pourgholi, A., Dadashpour, M., Mousapour, A., Firouzi Amandi, A., & Zarghami, N. (2021). Anticancer potential of silibinin loaded polymeric nanoparticles against breast cancer cells: Insight into the apoptotic genes targets. Asian Pacific Journal of Cancer Prevention: APJCP, 22(8), 2587–2596.
Mogheri, F., et al. (2021). Co-delivery of metformin and silibinin in dual-drug loaded nanoparticles synergistically improves chemotherapy in human non-small cell lung cancer A549 cells. Journal of Drug Delivery Science and Technology, 66, 102752.
Tousson, E., Salama, A. F., Dora, M. A., & Elony, M. A. (2016). Comparative cardioprotective effect of Egyptian Silybum marianum extract and Chinese silymarin in experimentally liver fibrosis. Journal of Bioscience and Applied Research, 2(2), 107–117.
Chen, S., et al. (2021). TPM3 mediates epithelial-mesenchymal transition in esophageal cancer via MMP2/MMP9. Annals of Translational Medicine, 9(16).
Huang, L., Guo, Z., Wang, F., & Fu, L. (2021). KRAS mutation: From undruggable to druggable in cancer. Signal Transduction and Targeted Therapy, 6(1), 386.
Jiang, H., & Li, H. (2021). Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: A systematic review and meta-analysis. BMC Cancer, 21, 1–13.
Author information
Authors and Affiliations
Contributions
A. Masoudi Chelegahi performed most of the experiments as part of his master’s degree in Genetics. R. Heidari and B. Karimi were thesis advisors and participated in intellectual discussions of the data. S. Reiisi coordinated the study, designed the experiments, and revised the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies on human or animals.
Consent for Publication
This article does not contain any individual person’s data in any form.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chelegahi, A.M., Reiisi, S., Heidari, R. et al. Novel Silymarin-Loaded Biosynthesized AgNPs for Improving Anticancer Activities in Breast Cancer. BioNanoSci. 13, 1817–1832 (2023). https://doi.org/10.1007/s12668-023-01183-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01183-1