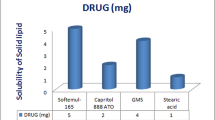
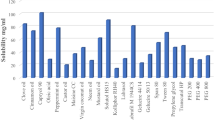
Abstract
The objective of the present research work was to enhance the bioavailability of dolutegravir sodium (anti-HIV drug) by loading the drug into a nanostructured lipid carrier (NLC), fabricated into the patch, and administered through a transdermal route. For this experimental work, six solid lipids, ten liquid lipids, and nine surfactants were selected for initial screening. The hot high-shear homogenization and melt emulsification low-temperature solidification methods were used to prepare nanostructured lipid carrier formulation. A Taguchi screening method was employed to find out the most influencing factors on critical quality attributes (CQAs) followed by a Box-Behnken design was applied to find the relationship between the independent variables and dependent variables. The selected optimized NLC formulation was characterized, and fabricated into the transdermal patch, and evaluated in a rabbit model for various pharmacokinetic parameters. The optimized formulation was characterized for particle size, zeta potential and polydispersity index (PDI), X-ray diffraction study, transmission electron microscopy analysis, in vitro drug release study, and in vivo pharmacokinetic parameter analysis. The results revealed that the particle size was 151.1 nm, zeta potential − 19.3 mV, and PDI was found to be 0.292. The AUC (0-∞) value of the drug-loaded NLC patch was 8156.426 ng h/ml, whereas 3945.696 ng h/ml and 4751.556 ng h/ml for pure drug and drug-loaded NLC formulation. From the experimental results, it was concluded that the NLC-loaded patch showed better bioavailability, which is nearly twice that of the pure drug administered by oral route. Hence, a fabricated NLC patch will be an assured drug delivery system for ameliorating the bioavailability of dolutegravir.
Graphical Abstract
















Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
World Health Organization report. https://www.who.int/data/gho/data/themes/hiv-aids. Accessed 23 Dec 2022
Anjum, R., & Lakshmi, P. K. (2020). Dolutegravir sodium loaded solid lipid nanoparticles: A vaginal drug delivery system for pre-exposure prophylaxis of HIV. J Res Pharm., 24(4), 552–561.
Venter, W. D. F., Clayden, P., & Serenata, C. (2017). The ADVANCE study: A groundbreaking trial to evaluate a candidate universal antiretroviral regimen. Current Opinion in HIV and AIDS., 12(4), 351–354. https://doi.org/10.1097/COH.0000000000000389
Shaikh, N. A., & Lala, R. R. (2021). Formulation development of dolutegravir sodium loaded nano lipid carriers for improved solubility and permeability. IJPSR., 12(7), 3654–3665.
Ghasemiyeh, P., & Mohammadi-Samani, S. (2018). Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res Pharm Sci., 13(4), 288–303.
Teng, Z., Yu, M., Ding, Y., Zhang, H., Shen, Y., Jiang, M., Liu, P., Opoku-Damoah, Y., Webster, T. J., & Zhou, J. (2019). Preparation and characterization of nimodipine-loaded nanostructured lipid systems for enhanced solubility and bioavailability. International Journal of Nanomedicine, 14, 119–133.
Brito Raj, S., Chandrasekhar, K. B., & Reddy, K. B. (2019). Formulation, in-vitro and in-vivo pharmacokinetic evaluation of simvastatin nanostructured lipid carrier loaded transdermal drug delivery system. Future Journal of Pharmaceutical Sciences., 5(9), 3–14.
Negi, L. M., Jaggi, M., & Talegaonkar, S. (2014). Development of protocol for screening the formulation components and the assessment of common quality problems of nano-structured lipid carriers. International Journal of Pharmaceutics, 461, 403–410.
Patel, D., Dasgupta, S., Sanjay Dey, Y., Ramani, R., Ray, S., & Mazumder, B. (2012). Nanostructured lipid carriers (NLC)-based gel for the topical delivery of aceclofenac: Preparation, characterization, and in vivo evaluation. Scientia Pharmaceutica, 80(3), 749–764. https://doi.org/10.3797/scipharm.1202-12
Kosnik, A., Szymanska, E., Czarnomysy, R., Jacyna, J., Markuszewski, M., Basa, A., & Winnicka, K. (2021). Nanostructured lipid carriers engineered as topical delivery of etodolac: Optimization and cytotoxicity studies. Materials., 14, 596. https://doi.org/10.3390/ma14030596
Shailendra, B., Jai Bharti, S., Ruchi, K., Manish, K., Vipin, S., & Shailendra, M. (2021). Design and optimization of febuxostat-loaded nano lipid carriers using full factorial design. Turk J Pharm Sci., 18(1), 61–67.
Cirri, M., Maestrini, L., Maestrelli, F., Mennini, N., Mura, P., Ghelardini, C., Di Cesare, L., & Mannelli,. (2018). Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Delivery, 25(1), 1910–1921.
Iqbal, B., & JavedAli, S. B. (2018). Silymarin loaded nanostructured lipid carrier: From design and dermatokinetic study to mechanistic analysis of epidermal drug deposition enhancement. Journal of Molecular Liquids, 255, 513–529.
Gaba, B., Fazil, M., Khan, S., Ali, A., Baboota, S., & Ali, J. (2015). Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bulletin of Faculty of Pharmacy. Cairo University., 53, 147–159.
Bahari, L. A. S., & Hamishehkar, H. (2016). The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; A comparative literature Review. Adv Pharm Bull., 6(2), 143–151.
Sharma, A., & Baldi, A. (2018). Nanostructured lipid carriers: A review. J Develop Drugs., 7(1), 1–12. https://doi.org/10.4172/2329-6631.1000191
Hanifiyah, I. A., Rosita, N., & Purwanti, T. (2021). Production method of nanostructured lipid carrier (NLC): Hot and cold homogenization against NLC-coenzyme Q10 characteristics. Journal of Computational and Theoretical Nanoscience., 18(1), 26–31. https://doi.org/10.1166/jctn.2021.9467
Singh, A. P., Sharma, S. K., Gaur, P. K., & Gupta, D. K. (2021). Fabrication of mupirocin-loaded nanostructured lipid carrier and its in vitro characterization. ASSAY and drug development technologies, 19(4), 216–225. https://doi.org/10.1089/adt.2020.1070
Jain, K., Sood, S., & Gowthamarajan, K. (2015). Optimization of artemether loaded NLC for intranasal delivery using central composite design. Drug Delivery, 22, 940–954.
Uprit, S., Sahu, R. K., Roy, A., & Pare, A. (2013). Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm J., 21(4), 379–385. https://doi.org/10.1016/j.jsps.2012.11.005
Shah, N. V., Seth, A. K., Balaraman, R., Aundhia, C. J., Maheshwari, R. A., & Parma, G. R. (2016). Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: Design and in vivo study. Journal of Advanced Research., 7, 423–434. https://doi.org/10.1016/j.jare.2016.03.002
Cirri, M., Bragagni, M., Mennini, N., & Mura, P. (2012). Development of a new delivery system consisting in ‘“drug–in cyclodextrin– in nanostructured lipid carriers”’ for ketoprofen topical delivery. European Journal of Pharmaceutics and Biopharmaceutics., 80, 46–53.
People PV, & Singh, K. K. (2011). Development and evaluation of colloidal modified nano lipid carrier: Application to topical delivery of tacrolimus. Eur J Pharm Biopharm., 79, 82–94.
Tran, T. H., Ramasamy, T., Truong, D. H., Choi, H.-G., Yong, C. S., & Kim, J. O. (2014). Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS Pharm SciTech., 15(6), 1509–1515. https://doi.org/10.1208/s12249-014-0175-y
Izham, M. N. M., Hussin, Y., Rahim, N. F. C., Aziz, M. N. M., Yeap, S. K., Rahman, H. S., Masarudin, M. J., Mohamad, N. E., Abdullah, R., & Alitheen, N. B. (2021). Physicochemical characterization, cytotoxic effect and toxicity evaluation of nanostructured lipid carrier loaded with eucalyptol. BMC Complement Med Ther., 21(254), 1–17. https://doi.org/10.1186/s12906-021-03422-y
Ortiz, A. C., Yanez, O., Salas-Huenuleo, E., & Morales, J. O. (2021). Development of a nanostructured lipid carrier (NLC) by a low-energy method, comparison of release kinetics and molecular dynamics simulation. Pharmaceutics., 13(4), 531. https://doi.org/10.3390/pharmaceutics13040531
Samiullah, UmerJan, Syed, Gul, Rahman, Jalaludin, Syed, & Asmathullah. (2020). Formulation and evaluation of transdermal patches of pseudoephedrine hcl. Int J App Pharm, 12(3), 121–127. https://doi.org/10.22159/ijap.2020v12i3.37080
Suryani, W. O. S. M., Ruslin, M. N., Rima, A., Marganita, H., et al. (2019). Formulation and physical characterization of curcumin nanoparticle transdermal patch. Int J Appl Pharm., 11, 217–221.
Pankaj Sharma and Mukul Tailang. (2022). Primaquine-loaded transdermal patch for treating malaria: Design, development, and characterization. Future Journal of Pharmaceutical Sciences., 8, 43. https://doi.org/10.1186/s43094-022-00433-5
Bazigha, K., Rasool, A., Mohammed, A. A., & Salem, Y. Y. (2021). The optimization of a dimenhydrinate transdermal patch formulation based on the quantitative analysis of in vitro release data by DDsolver through skin penetration studies. Scientia Pharmaceutica, 89(3), 33. https://doi.org/10.3390/scipharm89030033
Arunprasert, K., Pornpitchanarong, C., Piemvuthi, C., Siraprapapornsakul, S., Sripeangchan, S., Lertsrimongkol, O., Opanasopit, P., & Patrojanasophon, P. (2022). Nanostructured lipid carrier-embedded polyacrylic acid transdermal patches for improved transdermal delivery of capsaicin. European Journal of Pharmaceutical Sciences, 173, 106169. https://doi.org/10.1016/j.ejps.2022.106169
Chauhan, I., Yasi, M., Verma, M., & Singh, A. P. (2020). Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Adv Pharm Bull, 10(2), 150–165. https://doi.org/10.34172/apb.2020.021
Acknowledgements
The authors wish to acknowledge the Roland Institute of Pharmaceutical Sciences for providing facilities to carry out the research work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The conducted research work was approved by the IAEC (institutional animal ethical committee) with Reg. number 926/PO/Re/s/06 and approval no. 143.
Consent to Participate
The research study does not involve human subject.
Consent for Publication
This study does not contain any individual person’s data at any form.
Competing Interests
The authors declare no competing interests.
Animal Studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahoo, L., Jena, G.K., Patro, C.S. et al. In Vitro and In Vivo Characterization of Transdermal Patch Loaded with Nanostructured Lipid Carrier for Bioavailability Enhancement of Dolutegravir Sodium Using Taguchi and Box-Behnken Design. BioNanoSci. 13, 1213–1230 (2023). https://doi.org/10.1007/s12668-023-01143-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01143-9