Abstract
While most metal-based nanomaterials lack regulatory approval for clinical applications in pharmacology, the FDA-approved Feraheme (ferumoxytol) has been among the few exceptions. Further, the approval of several iron nanoparticles (NPs) in nanotherapeutics has inspired the need to develop a new immunoprotective biomaterial (BM) involving superparamagnetic iron oxide NPs. This article emphasizes the impact of superparamagnetic NPs on porphyrins in the capacity of intensifying their biological activities under biocompatible conditions. To explore the cancer photodynamic therapy (PDT) and post-PDT anti-inflammatory and immunoprotective significance in developing BMs as anti-cancer drugs, the photobiological significance of a hydrophilic BM of superparamagnetic Fe3O4 NP functionalized with a tri-pyridyl porphyrin photosensitizer (PS) through a flexible 4-phenylaminoacetic acid linker is investigated. Fluorescence confocal microscopy with spectral imaging indicated high uptake of the BM Fe3O4 NP-porphyrin (C-NP) in the human gastric cancer (AGS) cell line. The potential of the synthesized BM to promote apoptosis through the upregulation of p21 expression and sub-G0-G1 phase arrest of the cell cycle has been reported. Under PDT conditions, higher apoptosis rates were obtained by C-NP than its free base precursor C. The Fe3O4 NPs have been found to influence the porphyrin PS by enhancing their anti-inflammatory properties apparently by reducing nitric oxide (under light irradiation and in the dark), myeloperoxidase, and superoxide production in murine macrophages.
Graphical Abstract









Similar content being viewed by others
Data Availability
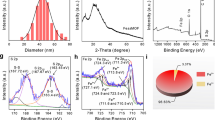
The Supporting Information (inclusive of characterization methods and experimental data) is available free of charge at www.
References
Henderson, B. W., & Dougherty, T. J. (1992). How does photodynamic therapy work? Photochemical & Photobiological Sciences, 55, 145–157.
Marcus, S. L., & McIntyre, W. R. (2002). Emerging Drugs, Photodynamic therapy systems and applications. Expert Opinion, 7, 321–334.
Dolmans, D. E., Fukumura, D., & Jain, R. K. (2003). Photodynamic therapy for cancer. Nature Reviews Cancer, 3, 380–387.
Ohtani, K., & Ikeda, N. (2016). Photodynamic therapy for lung cancer. Kyobu Geka Japanese Journal Thoracic Surgery Clinics, 69, 694–699.
Lucky, S. S., Soo, K. C., & Zhang, Y. (2015). Nanoparticles in photodynamic therapy. Chemical reviews, 115, 1990–2042.
Santiago-Raber, M.-L., Lawson, B. R., Dummer, W., Barnhouse, M., Koundouris, S., Wilson, C. B., Kono, D. H., & Theofilopoulos, A. N. (2001). Role of cyclin kinase inhibitor p21 in systemic autoimmunity. Journal of Immunology, 167, 4067–4074.
Abbas, T., & Dutta, A. (2009). p21 in cancer: Intricate networks and multiple activities. Nature Reviews Cancer, 9, 400–414.
Chan, W.-H. (2011). Photodynamic treatment induces an apoptotic pathway involving calcium, nitric oxide, p53, p21-activated kinase 2, and c-Jun N-terminal kinase and inactivates survival signal in human umbilical vein endothelial cells. International Journal of Molecular Sciences, 12, 1041–1059.
Nakayama, T., Kobayashi, T., Shimpei, O., Fukuhara, H., Namikawa, T., Inoue, K., Hanazaki, K., Takahashi, K., Nakajima, M., & Tanaka, T. (2019). Photoirradiation after aminolevulinic acid treatment suppresses cancer cell proliferation through the HO-1/p21 pathway. Photodiagnosis and Photodynamic Therapy, 28, 10–17.
Rehman, M. U., Rashid, S., Arafah, A., Qamar, W., Alsaffar, R. M., Ahmad, A., Almatroudi, N. M., Alqahtani, S., Rashid, S. M., & Ahmad, S. B. (2020). Piperine regulates Nrf-2/Keap-1 signalling and exhibits anticancer effect in experimental colon carcinogenesis in Wistar rats. Biology, 9, 302.
Woo, H. D., & Kim, J. (2013). Dietary flavonoid intake and risk of stomach and colorectal cancer. World Journal of Gastroenterology, 19, 1011.
Pollard, J. W. (2009). Trophic macrophages in development and disease. Nature Reviews Immunology, 9, 259–270.
Gordon, S. (2003). Alternative activation of macrophages. Nature Reviews Immunology, 3, 23–35.
Mantovani, A., Sozzani, S., Locati, M., Allavena, P., & Sica, A. (2002). Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology, 23, 549–555.
Ojalvo, L. S., King, W., Cox, D., & Pollard, J. W. (2009). High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. American Journal of Pathology, 174, 1048–1064.
Ojalvo, L. S., Whittaker, C. A., Condeelis, J. S., & Pollard, J. W. (2010). Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. Journal of Immunology, 184, 702–712.
G. Hu, M. Guo, J. Xu, F. Wu, J. Fan, Q. Huang, G. Yang, Z. Lv, X. Wang, Y. Jin (2019) Nanoparticles targeting macrophages as potential clinical therapeutic agents against cancer and inflammation. Frontiers in Immunology. 10
Vitale, I., Manic, G., Coussens, L. M., Kroemer, G., & Galluzzi, L. (2019). Macrophages and metabolism in the tumor microenvironment. Cell Metabolism, 30, 36–50.
Ghazanfari, M. R., Kashefi, M., Shams, S. F., & Jaafari, M. R. (2016). Perspective of Fe3O4 nanoparticles role in biomedical applications. Biochemistry Research International, 2016, 1–32.
Issa, B., Obaidat, I. M., Albiss, B. A., & Haik, Y. (2013). Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. International Journal of Molecular Sciences, 14, 21266–21305.
Liu, X., Jin, Y., Liu, T., Yang, S., Zhou, M., Wang, W., & Yu, H. (2020). Iron-based theranostic nanoplatform for improving chemodynamic therapy of cancer. ACS Biomaterials of Science and Engineering, 6, 4834–4845.
Bobo, D., Robinson, K. J., Islam, J., Thurecht, K. J., & Corrie, S. R. (2016). Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharmaceutical Research, 33, 2373–2387.
Yan, L., Amirshaghaghi, A., Huang, D., Miller, J., Stein, J. M., Busch, T. M., Cheng, Z., & Tsourkas, A. (2018). Protoporphyrin IX (PpIX)-coated superparamagnetic iron oxide nanoparticle (SPION) nanoclusters for magnetic resonance imaging and photodynamic therapy. Advanced Functional Materials, 28, 1707030.
Nafiujjaman, M., Revuri, V., Nurunnabi, M., Jae Cho, K., & Lee, Y.-K. (2015). Photosensitizer conjugated iron oxide nanoparticles for simultaneous in vitro magneto-fluorescent imaging guided photodynamic therapy. Chemical Communications, 51, 5687–5690.
Yan, L., Luo, L., Amirshaghaghi, A., Miller, J., Meng, C., You, T., Busch, T. M., Tsourkas, A., & Cheng, Z. (2019). Dextran-Benzoporphyrin Derivative (BPD) Coated superparamagnetic iron oxide nanoparticle (SPION) micelles for T2-weighted magnetic resonance imaging and photodynamic therapy. Bioconjugate Chemistry, 30, 2974–2981.
Bolfarini, G. C., Siqueira-Moura, M. P., Demets, G. J. F., Morais, P. C., & Tedesco, A. C. (2012). In vitro evaluation of combined hyperthermia and photodynamic effects using magnetoliposomes loaded with cucurbit[7]uril zinc phthalocyanine complex on melanoma. Journal of Photochemistry and Photobiology B Biology, 115, 1–4.
H. Gu, K. Xu, Z. Yang, C. K. Chang, B. Xu (2005) Synthesis and cellular uptake of porphyrin decorated iron oxide nanoparticles—a potential candidate for bimodal anticancer therapy. Chemical Communications 4270–4272.
Pellosi, D. S., Macaroff, P. P., Morais, P. C., & Tedesco, A. C. (2018). Magneto low-density nanoemulsion (MLDE): A potential vehicle for combined hyperthermia and photodynamic therapy to treat cancer selectively. Materials Science and Engineering: C, 92, 103–111.
Vieira Ferreira, L. F., Ferreira Machado, I., Gama, A., Lochte, F., Socoteanu, R. P., & Boscencu, R. (2020). A, Surface photochemical studies of nano-hybrids of A3B porphyrins and Fe3O4 silica-coated nanoparticles. Journal of Photochemistry and Photobiology, 387, 112152.
Penon, O., Marín, M. J., Amabilino, D. B., Russell, D. A., & Pérez-García, L. (2016). Iron oxide nanoparticles functionalized with novel hydrophobic and hydrophilic porphyrins as potential agents for photodynamic therapy. Journal of Colloid and Interface Science, 462, 154–165.
Mbakidi, J. P., Bregier, F., Ouk, T. S., Granet, R., Alves, S., Riviere, E., Chevreux, S., Lemercier, G., & Sol, V. (2015). Magnetic dextran nanoparticles that bear hydrophilic porphyrin derivatives: Bimodal agents for potential application in photodynamic therapy. Chempluschem, 80, 1416–1426.
Balivada, S., Rachakatla, R. S., Wang, H., Samarakoon, T. N., Dani, R. K., Pyle, M., Kroh, F. O., Walker, B., Leaym, X., Koper, O. B., Tamura, M., Chikan, V., Bossmann, S. H., & Troyer, D. L. (2010). A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer, 10, 119.
Zhang, H., Li, Y. H., Chen, Y., Wang, M. M., Wang, X. S., & Yin, X. B. (2017). Fluorescence and magnetic resonance dual-modality imaging-guided photothermal and photodynamic dual-therapy with magnetic porphyrin-metal organic framework nanocomposites. Science Reports, 7, 44153.
Rajkumar, S., & Prabaharan, M. (2017). Theranostics based on iron oxide and gold nanoparticles for imaging- guided photothermal and photodynamic therapy of cancer. Current Topics in Medicinal Chemistry, 17, 1858–1871.
Sengupta, D., Das, S., Sharma, D., Chattopadhyaya, S., Mukherjee, A., Mazumdar, Z. H., Das, B., Basu, S., & Sengupta, M. (2022). An anti-inflammatory Fe3O4-porphyrin nanohybrid capable of apoptosis through upregulation of p21 kinase inhibitor having immunoprotective properties under anticancer PDT conditions. ChemMedChem, 17, e202100550.
Xu, Z., Hou, Y., & Sun, S. (2007). Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. Journal of the American Chemical Society, 129, 8698–8699.
Gouterman, M. (1961). Spectra of porphyrins. Journal of Molecular Spectroscopy, 6, 138–163.
Antonangelo, A. R., Westrup, K. C. M., Burt, L. A., Bezzu, C. G., Malewschik, T., Machado, G. S., Nunes, F. S., McKeown, N. B., & Nakagaki, S. (2017). crystallographic characterization and homogeneous catalytic activity of novel unsymmetric porphyrins. RSC Advanced Synthesis, 7, 50610–50618.
Dechan, P., Devi Bajju, G., & Sood, P. (2020). Trans A2B2 porphyrins: Synthesis, crystal structure determinations and Hirshfeld surface analysis. ChemistrySelect, 5, 7298–7309.
D. Sengupta, (2006) University of Sydney
Aragón, F. H., Coaquira, J. A. H., Villegas-Lelovsky, L., da Silva, S. W., Cesar, D. F., Nagamine, L. C. C. M., Cohen, R., Menéndez-Proupin, E., & Morais, P. C. (2015). Evolution of the doping regimes in the Al-doped SnO2 nanoparticles prepared by a polymer precursor method. Journal of Physics: Condensed Matter, 27, 095301.
Li, Z., Wang, D., Xu, M., Wang, J., Hu, X., Anwar, S., Tedesco, A. C., Morais, P. C., & Bi, H. (2020). Fluorine-containing graphene quantum dots with a high singlet oxygen generation applied for photodynamic therapy. Journal of Materials Chemistry B, 8, 2598–2606.
Mikhaylova, M., Kim, D. K., Bobrysheva, N., Osmolowsky, M., Semenov, V., Tsakalakos, T., & Muhammed, M. (2004). Superparamagnetism of magnetite nanoparticles: Dependence on surface modification. Langmuir, 20, 2472–2477.
Kodama, R. H., Berkowitz, A. E., McNiff, E., Jr., & Foner, S. (1996). Surface spin disorder in NiFe 2 O 4 nanoparticles. Physical Review Letters, 77, 394–397.
Farhanian, D., De Crescenzo, G., & Tavares, J. R. (2018). Large-scale encapsulation of magnetic iron oxide nanoparticles via syngas photo-initiated chemical vapor deposition. Science Report, 8, 1–11.
Mahajan, P. G., Dige, N. C., Vanjare, B. D., Phull, A. R., Kim, S. J., Hong, S. K., & Lee, K. H. (2018). Synthesis, photophysical properties and application of new porphyrin derivatives for use in photodynamic therapy and cell imaging. Journal of Fluorescence, 28, 871–882.
Mahajan, P. G., Dige, N. C., Vanjare, B. D., Kim, C. H., Seo, S. Y., & Lee, K. H. (2020). Design and synthesis of new porphyrin analogues as potent photosensitizers for photodynamic therapy: Spectroscopic approach. Journal of Fluorescence, 30, 397–406.
Mahajan, P. G., Dige, N. C., Vanjare, B. D., Eo, S.-H., Seo, S.-Y., Kim, S. J., Hong, S.-K., Choi, C.-S., & Lee, K. H. (2019). A potential mediator for photodynamic therapy based on silver nanoparticles functionalized with porphyrin. Journal of Photochemistry and Photobiology A: Chemistry, 377, 26–35.
Sengupta, D., Mazumdar, Z. H., Mukherjee, A., Sharma, D., Halder, A. K., Basu, S., & Jha, T. (2018). Benzamide porphyrins with directly conjugated and distal pyridyl or pyridinium groups substituted to the porphyrin macrocycles: Study of the photosensitising abilities as inducers of apoptosis in cancer cells under photodynamic conditions. Journal of Photochemistry and Photobiology. B, Biology, 178, 228–236.
Sengupta, D., Timilsina, U., Mazumder, Z. H., Mukherjee, A., Ghimire, D., Markandey, M., Upadhyaya, K., Sharma, D., Mishra, N., & Jha, T. (2019). Dual activity of amphiphilic Zn (II) nitroporphyrin derivatives as HIV-1 entry inhibitors and in cancer photodynamic therapy. European Journal of Medicinal Chemistry, 174, 66–75.
Sharma, D., Mazumder, Z. H., Sengupta, D., Mukherjee, A., Sengupta, M., Das, R. K., Barbhuiya, M. H., Palit, P., & Jha, T. (2021). Cancer photocytotoxicity and anti-inflammatory response of cis-A2B2 type meso-p-nitrophenyl and p-hydroxyphenyl porphyrin and its zinc(II) complex: A synthetic alternative to the THPP synthon. New Journal of Chemistry, 45, 2060–2068.
Shimizu, S., Shinohara, Y., & Tsujimoto, Y. (2000). Bax and Bcl-x L independently regulate apoptotic changes of yeast mitochondria that require VDAC but not adenine nucleotide translocator. Oncogene, 19, 4309–4318.
Jiang, S., Cai, J., Wallace, D. C., & Jones, D. P. (1999). Cytochrome c-mediated apoptosis in cells lacking mitochondrial DNA signaling pathway involving release and caspase 3 activation is conserved. International Journal of Biological Chemistry, 274, 29905–29911.
Jo, W.-S., Jeong, M.-H., Jin, Y.-H., Jang, J.-Y., Nam, B.-H., Son, S.-H., Choi, S.-S., Yoo, Y.-H., Kang, C.-D., Lee, J.-D., & Jeong, S.-J. (2005). Loss of mitochondrial membrane potential and caspase activation enhance apoptosis in irradiated K562 cells treated with herbimycin A. International Journal of Radiation Biology, 81, 531–543.
Oh, J. M., Lee, J., Im, W. T., & Chun, S. (2019). Ginsenoside Rk1 induces apoptosis in neuroblastoma cells through loss of mitochondrial membrane potential and activation of caspases. International Journal of Molecular Sciences, 20, 1213–1230.
Liu, J., Zhao, Y., Shi, Z., & Bai, Y. (2019). Antitumor effects of helenalin in doxorubicin-resistant leukemia cells are mediated via mitochondrial mediated apoptosis, loss of mitochondrial membrane potential, inhibition of cell migration and invasion and downregulation of PI3-kinase/AKT/m-TOR signalling pathway. Journal of BUON, 24, 2068–2074.
Lam, T.-L., Tong, K.-C., Yang, C., Kwong, W.-L., Guan, X., Li, M.-D., Kar-Yan Lo, V., Lai-Fung Chan, S., Lee Phillips, D., Lok, C.-N., & C.-M,. (2019). Luminescent ruffled iridium(iii) porphyrin complexes containing N-heterocyclic carbene ligands: Structures, spectroscopies and potent antitumor activities under dark and light irradiation conditions. Chemical Science, 10, 293–309.
Karunakaran, S. C., Babu, P. S. S., Madhuri, B., Marydasan, B., Paul, A. K., Nair, A. S., Rao, K. S., Srinivasan, A., Chandrashekar, T. K., Rao, C. M., Pillai, R., & Ramaiah, D. (2019). In vitro demonstration of apoptosis mediated photodynamic activity and NIR nucleus imaging through a novel porphyrin. ACS Chemical Biology, 8, 127–132.
Ikeda, A., Satake, S., Mae, T., Ueda, M., Sugikawa, K., Shigeto, H., Funabashi, H., & Kuroda, A. (2017). Photodynamic activities of porphyrin derivative–cyclodextrin complexes by photoirradiation. ACS Medicinal Chemistry Letters, 8, 555–559.
Panieri, E., & Santoro, M. M. (2016). ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Disease, 7, e2253–e2253.
Valadez-Cosmes, P., Raftopoulou, S., Mihalic, Z. N., Marsche, G., & Kargl, J. (2022). Myeloperoxidase: Growing importance in cancer pathogenesis and potential drug target. Pharmacology and Therapeutics, 236, 108052.
Khan, A. A., Alsahli, M. A., & Rahmani, A. H. (2018). Myeloperoxidase as an active disease biomarker: Recent biochemical and pathological perspectives. Medical Sciences, 6, 33.
Jantan, I., Haque, M. A., Ilangkovan, M., & Arshad, L. (2019). Zerumbone from Zingiber zerumbet inhibits innate and adaptive immune responses in Balb/C mice. International Immunopharmacology, 73, 552–559.
Chi, D. S., Qui, M., Krishnaswamy, G., Li, C., & Stone, W. (2009). regulation of nitric oxide production from macrophages by lipopolysaccharide and catecholamines. Nitric oxide, 8, 127–132.
Babior, B. M. (1999). NADPH oxidase: An update. Blood, 93, 1464–1476.
Storz, P. (2005). Reactive oxygen species in tumor progression. Frontiers in Bioscience, 10, 1881–1896.
Liou, G. Y., & Storz, P. (2010). Reactive oxygen species in cancer. Free Radical Research, 44, 479–496.
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D., & Bitto, A. (2017). Oxidative stress: Harms and benefits for human health. Oxidative Medicine and Cellular Longevity, 2017, 1–13.
Huang, X., Chen, J., Wu, W., Yang, W., Zhong, B., Qing, X., & Shao, Z. (2020). Delivery of MutT homolog 1 inhibitor by functionalized graphene oxide nanoparticles for enhanced chemo-photodynamic therapy triggers cell death in osteosarcoma. Acta biomaterialia, 109, 229–243.
Reina, G., Peng, S., Jacquemin, L., Andrade, A. F., & Bianco, A. (2020). Hard nanomaterials in time of viral pandemics. ACS nano, 14, 9364–9388.
Anselmo, A. C., & Mitragotri, S. (2019). Nanoparticles in the clinic: An update. Bioengineering of Translational Medicine, 4, e10143.
Huang, Y., Hsu, J. C., Koo, H., & Cormode, D. P. (2022). Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics, 12, 796–816.
Bahadar, H., Maqbool, F., Niaz, K., & Abdollahi, M. (2016). Toxicity of nanoparticles and an overview of current experimental models. Iranian Biomedical Journal, 20, 1–11.
Egbuna, C., Parmar, V. K., Jeevanandam, J., Ezzat, S. M., Patrick-Iwuanyanwu, K. C., Adetunji, C. O., Khan, J., Onyeike, E. N., Uche, C. Z., Akram, M., Ibrahim, M. S., El Mahdy, N. M., Awuchi, C. G., Saravanan, K., Tijjani, H., Odoh, U. E., Messaoudi, M., Ifemeje, J. C., Olisah, M. C., … Ibeabuchi, C. G. (2021). Nanotoxicology. Journal of Toxicology, Toxicity of nanoparticles in biomedical application, 2021, 9954443.
Piktel, E., Niemirowicz, K., Wątek, M., Wollny, T., Deptuła, P., & Bucki, R. (2016). Recent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapy. Journal of Nanobiotechnology, 14, 39.
Acknowledgements
STIC-SAIF Kochi is acknowledged for the NMR; we thank Dr. Ramkrishna Laha, Postdoctoral Associate, Ernest Mario School of Pharmacy, Rutgers University, New Brunswick, for helping us with the mass spectra. The authors further acknowledge the help and guidance received from Prof. Subrata Banerjee, Ex-professor and Head, Biophysics & Structural Genomics Division, Saha Institute of Nuclear Physics (SINP), Kolkata; Dr. Yashmin Choudhury, Department of Biotechnology, Assam University, Silchar, India; and Dr. Aviva Levina, School of Chemistry, The University of Sydney, Australia.
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript. DS conceived, designed, and standardized the experiments by initially synthesizing the precursor porphyrins. DS synthesized the nanoparticles and functionalized them along with SD and ZHM. The biological studies were carried out by AM and SC at the Department of Zoology, Charuchandra College, Kolkata 700 029, India, and the School of Biological Sciences, Ramkrishna Mission Vivekananda Educational & Research Institute (RKMVERI), Narendrapur, Kolkata 700 103, India. MS and BD performed the immunomodulatory experiments on macrophage cell line at the Department of Biotechnology, Assam University, Silchar 788 011, India. AM conducted the photobiological assays related to ROS and GSH in AGS cell line. PP helped with the interpretation of the results of the photobiological assays along with DS and AM. DS performed the Alder’s synthesis under various conditions, performed different methods of isolation and characterization of the various porphyrin derivatives at Assam University, Silchar (AUS), and performed lifetime emission experiments along with SB at Saha Institute of Nuclear Physics, Kolkata 700 064, India.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Research Involving Humans and Animals Statement
As investigators, we conducted animal research ethically and humanely by using the minimum number of animals needed for scientifically valid results and ensuring appropriate housing, feeding, and sanitation. We administered appropriate anesthetics, analgesics, or tranquilizers to minimize any pain and discomfort, and obtained approval and oversight from an institutional animal experiment committee.
Consent for Publication
All the authors have reviewedand registered their consent to the final version of the manuscript.
Informed Consent
Our work did not involve any experiments on human subjects, as such, informed consent is not applicable to the current research work.
Funding Statement
This work was supported by the DBT project (Reg. No. BT/PR25024/NER/95/961/2017) granted to Devashish Sengupta by the Department of Biotechnology, Ministry of Science and Technology, Government of India.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, D., Das, S., Mazumdar, Z.H. et al. A Photoactive Magnetic Nanoparticle-Porphyrin Biomaterial Capable of Upregulation of Cancer PDT Having a Concomitant Immune Signature in Noncancerous Cells. BioNanoSci. 13, 625–637 (2023). https://doi.org/10.1007/s12668-023-01104-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01104-2