Abstract
Calcium phosphate (CaP) is a kind of eco-friendly biodegradable material chemically similar to human hard tissue like bone and teeth and is highly biocompatible. CaPs have excellent biological quality, are cheap and easy to produce, are safe, and may be approved for clinical applications quite fast. CaP materials have proven themselves, yet their future is still bright. Today’s major global public health issue is the prevalence of injury and illness brought on by bone fractures. There are a number of problems with the current treatments that call for research into better methods of dealing with bone disorders. Gene therapy has recently gained attention as a promising strategy for efficient bone repair and regeneration via the use of RNA interference (RNAi) systems to modulate gene expression in the bone microenvironment. CaP nanoparticles have been shown to be efficient transporters for biomolecules that cannot enter cells to exert their biological impacts, such as nucleic acids, proteins, peptides, antibodies, and medicines. Surface functionalization and incorporation of cargo molecules make these nanoparticles distinct from their solid counterparts. This protects against nucleases and allows for selective cellular targeting. In this overview, we looked at the fundamentals of nanoparticles of calcium phosphates, including their production techniques, physicochemical features, and applications in transfection, gene silencing, drug administration, the environment, electricity, tissue engineering, drug delivery, and biomedicine. This study is predicted to be extremely valuable and advantageous to the researcher in the future progress of the research work in therapeutic applications since it comprises very comprehensive thoughts about calcium phosphate nanoparticles.
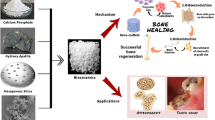
Graphical Abstract
Calcium phosphate is known to be a “super material” with improved biological qualities such as bioactivity, biodegradability, cellular function, and osteoconduction. The synthesis and fabrication of calcium phosphate by different crucial techniques in enhancing the physical and chemical properties allow it to be potential in various bioengineering fields such as drug delivery, bone regeneration, gene silencing, and electrical as well as environmental applications.






Similar content being viewed by others
Data Availability
Data inculded in the journal has been properly supported by appropriate references. Although all the figures and schemes in the article has been prepared by our own.
Abbreviations
- ATP:
-
Adenosine triphosphate
- ADP:
-
Adenosine diphosphate
- CaP:
-
Calcium phosphate
- PCBs:
-
Polychlorinated biphenyls
- NZVI:
-
Nanoscale zero-valent iron
- LED:
-
Light-emitting diode
- MRI:
-
Magnetic resonance imaging
- PET:
-
Positron emission tomography
- DNA:
-
Deoxyribonucleic acid
- UV:
-
Ultraviolet
- SAXS:
-
Small-angle X-ray scattering
- SANS:
-
Small-angle neutron scattering
- GI:
-
Grazing incidence
- XR:
-
X-ray
- NR:
-
Neutron reflectometry
- MCPM:
-
Monocalcium phosphate monohydrate
- DCPD:
-
Dicalcium phosphate dihydrate
- SSA:
-
Specific surface area
- HA:
-
Hydroxyapatite
- TCP:
-
Tricalcium phosphate
- DoE:
-
Design of experiment
- NPs:
-
Nanoparticles
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
- XRD:
-
X-ray diffraction
- BET:
-
Brunauer-Emmett-Teller
- DLS:
-
Dynamic light scattering
- TGA:
-
Thermo gravimetric analyzer
- IAP:
-
Ion activity products
- Ca:
-
Calcium
- P:
-
Phosphate
- TRITC:
-
Tetramethylrhodamine isothiocyanate
- BSA:
-
Bovine serum albumin
- eGFP:
-
Enhanced green fluorescent protein
- RISC:
-
RNA-induced silencing complex
- HeLa:
-
Human cervix epithelial cells
- PDT:
-
Photodynamic therapy
- mTHPP:
-
5,10,15,20-Tetrakis(3-hydroxyphenyl) porphyrin
- WSL:
-
White spots lesion
- ACP:
-
Amorphous calcium phosphate
- PEG:
-
Polyethylene glycol
- PLGA:
-
Poly (lactic-co-glycolic acid)
- PCL:
-
Polycaprolactone
- PLLA:
-
Poly (l-lactic acid)
References
Dorozhkin, S. V. (2013). A detailed history of calcium orthophosphates from 1770s till 1950. Materials Science and Engineering C, 33, 3085–3110.
Forsen, S. T. U. R. E., & Kordel, J. O. H. A. N. (1994). Calcium in biological systems (pp. 107–166). Bioinorganic chemistry.
Yang, R. N., Ye, F., Cheng, L. J., Wang, J. J., Lu, X. F., Shi, Y. J., et al. (2011). Osteoinduction by Ca-P biomaterials implanted into the muscles of mice. Journal of Zhejiang University. Science. B, 12, 582–590.
Huang, L., Zhou, B., Wu, H., Zheng, L., & Zhao, J. (2017). Effect of apatite formation of biphasic calcium phosphate ceramic (BCP) on osteoblastogenesis using simulated body fluid (SBF) with or without bovine serum albumin (BSA). Materials Science and Engineering C, 70, 955–961.
Lu, J., Yu, H., & Chen, C. (2018). Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Advances, 8, 2015–2033.
Duan, R., Barbieri, D., Luo, X., Weng, J., de Bruijn, J. D., & Yuan, H. (2016). Submicron-surface structured tricalcium phosphate ceramic enhances the bone regeneration in canine spine environment. Journal of Orthopaedic Research, 34, 1865–1873.
Schilling, A. F., Linhart, W., Filke, S., Gebauer, M., Schinke, T., Rueger, J. M., & Amling, M. (2004). Resorbability of bone substitute biomaterials by human osteoclasts. Biomaterials, 25, 3963–3972.
Chiba, S., Anada, T., Suzuki, K., Saito, K., Shiwaku, Y., Miyatake, N., Suzuki, O., et al. (2016). Effect of resorption rate and osteoconductivity of biodegradable calcium phosphate materials on the acquisition of natural bone strength in the repaired bone. Journal of Biomedical Materials Research. Part A, 104, 2833–2842.
Lei, Y., Xu, Z., Ke, Q., Yin, W., Chen, Y., Zhang, C., & Guo, Y. (2017). Strontium hydroxyapatite/chitosan nanohybrid scaffolds with enhanced osteoinductivity for bone tissue engineering. Materials Science and Engineering C, 72, 134–142.
Fihri, A., Len, C., Varma, R. S., & Solhy, A. (2017). Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coordination Chemistry Reviews, 347, 48–76.
Bose, S., & Tarafder, S. (2012). Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomaterialia, 8, 1401–1421.
Carrodeguas, R. G., & De Aza, S. (2011). α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomaterialia, 7, 3536–3546.
Dorozhkin, S. V., & Epple, M. (2002). Biological and medical significance of calcium phosphates. Angewandte Chemie International Edition, 41, 3130–3146.
Nogi, K., Naito, M., & Yokoyama, T. (2012). Nanoparticle technology handbook. Elsevier.
Rotello, V. (Ed.). (2004). Nanoparticles: Building blocks for nanotechnology. Springer Science & Business Media.
Ghosh, S., & Ray, A. (2014). Alkyl chain length asymmetry effects of mixed n-acyl sarcosinate and N-cetylpyridinium chloride surfactants: Spontaneous formation of stable nanovesicles as excipient. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 461, 248–257.
Ringu, T., Ghosh, S., Das, A., & Pramanik, N. (2022). Zinc oxide nanoparticles: An excellent biomaterial for bioengineering applications. emergent mater., 1–20 https://doi.org/10.1007/s00289-022-04443-4
Ghosh, S., Ray, A., & Pramanik, N. (2020). Self-assembly of surfactants: An overview on general aspects of amphiphiles. Biophysical Chemistry, 265, 106429.
Fan, D., Chen, S., Johnson, R. L., & Tratnyek, P. G. (2015). Field deployable chemical redox probe for quantitative characterization of carboxymethylcellulose modified nano zerovalent iron. Environmental Science and Technology, 49, 10589–10597.
Ashraf, M. A., Peng, W., Zare, Y., & Rhee, K. Y. (2018). Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Research Letters, 13, 1–7.
Sadasivuni, K. K., Rattan, S., Waseem, S., Brahme, S. K., Kondawar, S. B., Ghosh, Mazumdar, P. et al. (2019). Silver nanoparticles and its polymer nanocomposites-Synthesis, optimization, biomedical usage, and its various applications. Polymer nanocomposites in biomedical engineering, London, United Kingdom, pp 331–373.
Wang, L., Hu, C., & Shao, L. (2017). The antimicrobial activity of nanoparticles: Present situation and prospects for the future. International Journal of Nanomedicine, 12, 1227.
Kausar, A. (2020). Flame retardant potential of clay nanoparticles (pp. 169–184). In Clay Nanoparticles.
Yang, D. (2012). Application of nanocomposites for supercapacitors: Characteristics and properties. Nanocomposites - New Trends and Developments, pp 299–328.
Bhardwaj, P., Singh, B., & Behera, S. P. (2021). Green approaches for nanoparticle synthesis: Emerging trends. Nanomaterials, pp167–193.
Vergaz, R., Algorri, J. F., Cuadrado, A., Sánchez-Pena, J. M., & García-Cámara, B. (2016). Control of the light interaction in a semiconductor nanoparticle dimer through scattering directionality. IEEE Photonics Journal, 8, 1–10.
Ghosh, S., Ghosh, S., Atta, A. K., & Pramanik, N. (2018). A succinct overview of hydroxyapatite based nanocomposite biomaterials: Fabrications, physicochemical properties and some relevant biomedical applications. J. Bionanoscience, 12, 143–158.
Ghosh, S., Raju, R. S. K., Ghosh, N., Chaudhury, K., Ghosh, S., Banerjee, I., & Pramanik, N. (2019). Development and physicochemical characterization of doxorubicin-encapsulated hydroxyapatite–polyvinyl alcohol nanocomposite for repair of osteosarcoma-affected bone tissues. Comptes Rendus Chimie, 22, 46–57.
Ghosh, S., Ghosh, S., Jana, S. K., & Pramanik, N. (2020). Biomedical application of doxorubicin coated hydroxyapatite—poly (lactide-co-glycolide) nanocomposite for controlling osteosarcoma therapeutics. Journal of Nanoscience and Nanotechnology, 20, 3994–4004.
Ghosh, S., Ghosh, S., & Pramanik, N. (2020). Bio-evaluation of doxorubicin (DOX)-incorporated hydroxyapatite (HAp)-chitosan (CS) nanocomposite triggered on osteosarcoma cells. Adv Compos Hybrid Mater, 3, 303–314.
Kumar, C. S., & Mohammad, F. (2011). Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Advanced Drug Delivery Reviews, 63, 789–808.
Mourdikoudis, S., Pallares, R. M., & Thanh, N. T. (2018). Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale, 10, 12871–12934.
Sadik, O. A. (2013). Anthropogenic nanoparticles in the environment. Environmental Science. Processes & Impacts, 15, 19–20.
Hirano, S. (2009). A current overview of health effect research on nanoparticles. Environmental Health and Preventive Medicine, 14, 223–225.
Al-Sanabani, J. S., Madfa, A. A., & Al-Sanabani, F. A. (2013). Application of calcium phosphate materials in dentistry. International Journal of Biomaterials.
Kokubo, T., Kushitani, H., Sakka, S., Kitsugi, T., & Yamamuro, T. (1990). Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. Journal of Biomedical Materials Research, 24, 721–734.
Hench, L. L., Day, D. E., Höland, W., & Rheinberger, V. M. (2010). Glass and medicine. International Journal of Applied Glass Science, 1, 104–117.
Schilling, A. F., Filke, S., Brink, S., Korbmacher, H., Amling, M., & Rueger, J. M. (2006). Osteoclasts and biomaterials. European Journal of Trauma and Emergency Surgery, 32, 107–113.
Tan, L., Yu, X., Wan, P., & Yang, K. (2013). Biodegradable materials for bone repairs: A review. Journal of Materials Science and Technology, 29, 503–513.
Doi, Y., Iwanaga, H., Shibutani, T., Moriwaki, Y., & Iwayama, Y. (1999). Osteoclastic responses to various calcium phosphates in cell cultures. Journal of Biomedical Materials Research, 47, 424–433.
Guo, X., Gough, J. E., Xiao, P., Liu, J., & Shen, Z. (2007). Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. Journal of Biomedical Materials Research. Part A, 82, 1022–1032.
Liu, H., & Webster, T. J. (2007). Nanomedicine for implants: A review of studies and necessary experimental tools., 28, 354–369.
Kumar, G., Waters, M. S., Farooque, T. M., Young, M. F., & Simon, C. G., Jr. (2012). Freeform fabricated scaffolds with roughened struts that enhance both stem cell proliferation and differentiation by controlling cell shape. Biomaterials, 33, 4022–4030.
Teixeira, S., Fernandes, M. H., Ferraz, M. P., & Monteiro, F. J. (2010). Proliferation and mineralization of bone marrow cells cultured on macroporous hydroxyapatite scaffolds functionalized with collagen type I for bone tissue regeneration. Journal of Biomedical Materials Research. Part A, 95, 1–8.
Zhuang, Z., Fujimi, T. J., Nakamura, M., Konishi, T., Yoshimura, H., & Aizawa, M. (2013). Development of a, b-plane-oriented hydroxyapatite ceramics as models for living bones and their cell adhesion behavior. Acta Biomaterialia, 9, 6732–6740.
Carbajal, L., Serena, S., Caballero, A., Saínz, M. A., Detsch, R., & Boccaccini, A. R. (2014). Role of ZnO additions on the β/α phase relation in TCP based materials: Phase stability, properties, dissolution and biological response. Journal of the European Ceramic Society, 34, 1375–1385.
Cheng, L., Shi, Y., Ye, F., & Bu, H. (2013). Osteoinduction of calcium phosphate biomaterials in small animals. Materials Science and Engineering C, 33, 1254–1260.
Habibovic, P., Yuan, H., Van Der Valk, C. M., Meijer, G., van Blitterswijk, C. A., & De Groot, K. (2005). 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials, 26, 3565–3575.
Habibovic, P., Sees, T. M., van den Doel, M. A., van Blitterswijk, C. A., & de Groot, K. (2006). Osteoinduction by biomaterials—Physicochemical and structural influences. Journal of Biomedical Materials Research. Part A, 77, 747–762.
Cheng, L., Ye, F., Yang, R., Lu, X., Shi, Y., Li, L., ... & Bu, H. (2010). Osteoinduction of hydroxyapatite/β-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomaterialia, 6 1569–1574.
Diaz-Flores, L. U. C. I. O., Gutierrez, R. I. C. A. R. D. O., Lopez-Alonso, A. N. T. O. N. I. O., Gonzalez, R. I. C. A. R. D. O., & Varela, H. I. L. D. A. (1992). Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clinical Orthopaedics and Related Research, 275, 280–286.
Chow, L. C., & Eanes, E. D. (2001). Solubility of calcium phosphates. Monographs in Oral Science, pp 94–111.
Deo, K. A., Lokhande, G., & Gaharwar, A. K. (2019). Nanostructured hydrogels for tissue engineering and regenerative medicine. Encyclopedia of Tissue Eng Regen Med, 1, 3.
Mehdawi, I. M., & Young, A. (2013). Antibacterial composite restorative materials for dental applications (pp. 270–293). Woodhead Publishing.
Song, M., Park, J., Lee, J., Suh, H., Lee, H., Ryu, D., & Lee, C. (2020). New analytical approach for the determination of calcium phosphate dibasic and tribasic in processed food by comparison of ion chromatography with high-performance liquid chromatography. Foods, 9, 248.
Mulye, N. V., & Turco, S. J. (1994). Use of dicalcium phosphate dihydrate for sustained release of highly water soluble drugs. Drug Development and Industrial Pharmacy, 20, 2621–2632.
Adawy, A., EL–BASSYOUNI, G. T., Ibrahim, M., & ABDEL–FATTAH, W. I. (2013). Bio nano material: The third alternative. Nanotechnology: Diagnostics and Therapeutics; Eds, 7, 27.
Bose, S., Tarafder, S., Edgington, J., & Bandyopadhyay, A. (2011). Calcium phosphate ceramics in drug delivery. JOM Journal of the Minerals Metals and Materials Society, 63, 93–98.
Arcos, D., Boccaccini, A. R., Bohner, M., Díez-Pérez, A., Epple, M., Gómez-Barrena, E., ... & Vallet-Regí, M. (2014). The relevance of biomaterials to the prevention and treatment of osteoporosis. Acta Biomaterialia, 10:1793–1805.
Da Silva Brum, I., De Carvalho, J. J., Da Silva Pires, J. L., De Carvalho, M. A. A., Dos Santos, L. B. F., & Elias, C. N. (2019). Nanosized hydroxyapatite and β-tricalcium phosphate composite: Physico-chemical, cytotoxicity, morphological properties and in vivo trial. Science and Reports, 9, 1–10.
Miernicki, M., Hofmann, T., Eisenberger, I., von der Kammer, F., & Praetorius, A. (2019). Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nature Nanotechnology, 14, 208–216.
Salma, K., Berzina-Cimdina, L., & Borodajenko, N. (2010). Calcium phosphate bioceramics prepared from wet chemically precipitated powders. Process. Appl. Ceram, 4, 45–51.
Welzel, T., Meyer-Zaika, W., & Epple, M. (2004). Continuous preparation of functionalised calcium phosphate nanoparticles with adjustable crystallinity. ChemComm, 10, 1204–1205.
Doll, T. A., Raman, S., Dey, R., & Burkhard, P. (2013). Nanoscale assemblies and their biomedical applications. Journal of the Royal Society, Interface, 10, 20120740.
Mohn, D., Doebelin, N., Tadier, S., Bernabei, R. E., Luechinger, N. A., Stark, W. J., & Bohner, M. (2011). Reactivity of calcium phosphate nanoparticles prepared by flame spray synthesis as precursors for calcium phosphate cements. Journal of Materials Chemistry, 21, 13963–13972.
Sokolova, V. V., Radtke, I., Heumann, R., & Epple, M. (2006). Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials, 27, 3147–3153.
Sadat-Shojai, M., Khorasani, M. T., Dinpanah-Khoshdargi, E., & Jamshidi, A. (2013). Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomaterialia, 9, 7591–7621.
Neira, I. S., Kolen’ko, Y. V., Lebedev, O. I., Van Tendeloo, G., Gupta, H. S., Guitián, F., & Yoshimura, M. (2009). An effective morphology control of hydroxyapatite crystals via hydrothermal synthesis. Crystal Growth & Design, 9, 466–474.
Ganesan, K., Kovtun, A., Neumann, S., Heumann, R., & Epple, M. (2008). Calcium phosphate nanoparticles: Colloidally stabilized and made fluorescent by a phosphate-functionalized porphyrin. Journal of Materials Chemistry, 18, 3655–3661.
Dördelmann, G., Kozlova, D., Karczewski, S., Lizio, R., Knauer, S., & Epple, M. (2014). Calcium phosphate increases the encapsulation efficiency of hydrophilic drugs (proteins, nucleic acids) into poly (d, l-lactide-co-glycolide acid) nanoparticles for intracellular delivery. Journal Materials Chemistry B, 2, 7250–7259.
Ataol, S., Tezcaner, A., Duygulu, O., Keskin, D., & Machin, N. E. (2015). Synthesis and characterization of nanosized calcium phosphates by flame spray pyrolysis, and their effect on osteogenic differentiation of stem cells. Journal of Nanoparticle Research, 17, 1–14.
Boutinguiza, M., Comesaña, R., Lusquiños, F., Riveiro, A., & Pou, J. (2011). Production of nanoparticles from natural hydroxylapatite by laser ablation. Nanoscale Research Letters, 6, 1–5.
Karlinsey, R. L., & Mackey, A. C. (2009). Solid-state preparation and dental application of an organically modified calcium phosphate. Journal of Materials Science, 44, 346–349.
Pedraza, C. E., Bassett, D. C., McKee, M. D., Nelea, V., Gbureck, U., & Barralet, J. E. (2008). The importance of particle size and DNA condensation salt for calcium phosphate nanoparticle transfection. Biomaterials, 29, 3384–3392.
Cheng, X., & Kuhn, L. (2007). Chemotherapy drug delivery from calcium phosphate nanoparticles. International Journal of Nanomedicine, 2, 667–674.
Das, A., Ringu, T., Ghosh, S., & Pramanik, N. (2022). A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polymer Bulletin. https://doi.org/10.1007/s00289-022-04443-4
Morgan, T. T., Muddana, H. S., Altinoglu, E. I., Rouse, S. M., Tabakovic, A., Tabouillot, T., & Adair, J. H. (2008). Encapsulation of organic molecules in calcium phosphate nanocomposite particles for intracellular imaging and drug delivery. Nano Letters, 8, 4108–4115.
Altınogˇlu, E. I., Russin, T. J., Kaiser, J. M., Barth, B. M., Eklund, P. C., Kester, M., & Adair, J. H. (2008). Near-infrared emitting fluorophore-doped calcium phosphate nanoparticles for in vivo imaging of human breast cancer. ACS Nano, 2, 2075–2084.
Isobe, T., Nakamura, S., Nemoto, R., Senna, M., & Sfihi, H. (2002). Solid-state double nuclear magnetic resonance study of the local structure of calcium phosphate nanoparticles synthesized by a wet-mechanochemical reaction. The Journal of Physical Chemistry B, 106, 5169–5176.
Liu, D. M., Troczynski, T., & Tseng, W. J. (2001). Water-based sol–gel synthesis of hydroxyapatite: Process development. Biomaterials, 22, 1721–1730.
Nayak, A. K. (2010). Hydroxyapatite synthesis methodologies: An overview. International Journal of ChemTech Research, 2, 903–907.
Chen, Q. Z., Wong, C. T., Lu, W. W., Cheung, K. M. C., Leong, J. C. Y., & Luk, K. D. K. (2004). Strengthening mechanisms of bone bonding to crystalline hydroxyapatite in vivo. Biomaterials, 25, 4243–4254.
Jordan, M., & Wurm, F. (2004). Transfection of adherent and suspended cells by calcium phosphate. Methods, 33, 136–143.
Oyane, A., Wang, X., Sogo, Y., Ito, A., & Tsurushima, H. (2012). Calcium phosphate composite layers for surface-mediated gene transfer. Acta Biomaterialia, 8, 2034–2046.
Lin, K., Wu, C., & Chang, J. (2014). Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomaterialia, 10, 4071–4102.
Chowdhury, E. H., Kunou, M., Nagaoka, M., Kundu, A. K., Hoshiba, T., & Akaike, T. (2004). High-efficiency gene delivery for expression in mammalian cells by nanoprecipitates of Ca–Mg phosphate. Gene, 341, 77–82.
Perez-Coronado, A. M., Calvo, L., Alonso-Morales, N., Heras, F., Rodriguez, J. J., & Gilarranz, M. A. (2016). Multiple approaches to control and assess the size of Pd nanoparticles synthesized via water-in-oil microemulsion. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 497, 28–34.
Zhou, W. Y., Wang, M., Cheung, W. L., Guo, B. C., & Jia, D. M. (2008). Synthesis of carbonated hydroxyapatite nanospheres through nanoemulsion. Journal of Materials Science. Materials in Medicine, 19, 103–110.
Koumoulidis, G. C., Katsoulidis, A. P., Ladavos, A. K., Pomonis, P. J., Trapalis, C. C., Sdoukos, A. T., & Vaimakis, T. C. (2003). Preparation of hydroxyapatite via microemulsion route. Journal of Colloid and Interface Science, 259, 254–260.
Jarcho, M., Salsbury, R. L., Thomas, M. B., & Doremus, R. H. (1979). Synthesis and fabrication of β-tricalcium phosphate (whitlockite) ceramics for potential prosthetic applications. Journal of Materials Science, 14, 142–150.
Puleo, D. A., Holleran, L. A., Doremus, R. H., & Bizios, R. (1991). Osteoblast responses to orthopedic implant materials in vitro. Journal of Biomedical Materials Research, 25, 711–723.
Wagner, V., Dullaart, A., Bock, A. K., & Zweck, A. (2006). The emerging nanomedicine landscape. Nature Biotechnology, 24, 1211–1217.
Wang, P., Lombi, E., Zhao, F. J., & Kopittke, P. M. (2016). Nanotechnology: A new opportunity in plant sciences. Trends in Plant Science, 21, 699–712.
Dorozhkin, S. V. (2010). Bioceramics of calcium orthophosphates. Biomaterials, 31, 1465–1485.
Uskoković, V. (2020). Ion-doped hydroxyapatite: An impasse or the road to follow. Ceramics International, 46, 11443–11465.
Victor, S. P., & Kumar, T. S. (2008). Tailoring calcium-deficient hydroxyapatite nanocarriers for enhanced release of antibiotics. Journal of Biomedical Nanotechnology, 4, 203–209.
Ali, I. (2012). New generation adsorbents for water treatment. Chemical Reviews, 112, 5073–5091.
Rhee, S. H. (2002). Synthesis of hydroxyapatite via mechanochemical treatment. Biomaterials, 23, 1147–1152.
Kim, I. S., & Kumta, P. N. (2004). Sol–gel synthesis and characterization of nanostructured hydroxyapatite powder. Materials Science and Engineering B, 111, 232–236.
Drouet, C., Bosc, F., Banu, M., Largeot, C., Combes, C., Dechambre, G., ... & Rey, C. (2009). Nanocrystalline apatites: From powders to biomaterials. Powder Technol, 190:118–122.
Jevtic, M., Mitric, M., Skapin, S., Jancar, B., Ignjatovic, N., & Uskokovic, D. (2008). Crystal structure of hydroxyapatite nanorods synthesized by sonochemical homogeneous precipitation. Crystal Growth & Design, 8, 2217–2222.
Di Chen, J., Wang, Y. J., Wei, K., Zhang, S. H., & Shi, X. T. (2007). Self-organization of hydroxyapatite nanorods through oriented attachment. Biomaterials, 28, 2275–2280.
Afshar, A., Ghorbani, M., Ehsani, N., Saeri, M. R., & Sorrell, C. C. (2003). Some important factors in the wet precipitation process of hydroxyapatite. Materials and Design, 24, 197–202.
Kim, D. W., Cho, I. S., Kim, J. Y., Jang, H. L., Han, G. S., Ryu, H. S., Hong, K. S., et al. (2010). Simple large-scale synthesis of hydroxyapatite nanoparticles: In situ observation of crystallization process. Langmuir, 26, 384–388.
Chen, J., Zheng, C., & Chen, G. A. (1996). Interaction of macro-and micromixing on particle size distribution in reactive precipitation. Chemical Engineering Science, 51, 1957–1966.
Kazmierczak, T., Schuttringer, E., Tomažić, B., & Nancollas, G. H. (1981). Controlled composition studies of calcium carbonate and sulfate crystal growth. Croatica Chemica Acta, 54, 277–287.
Uskokovic, V. (2009). Challenges for the modern science in its descend towards nano scale. Current Nanoscience, 5, 372–389.
Latocha, J., Wojasiński, M., Sobieszuk, P., & Ciach, T. (2018). Synthesis of hydroxyapatite in a continuous reactor: A review. Chemical and Process Engineering, 39(3), 281–293.
Massart, D. L., Vandeginste, B. G., Buydens, L. M., Lewi, P. J., Smeyers-Verbeke, J., & Jong, S. D. (1998). Handbook of chemometrics and qualimetrics. Elsevier Science Inc.
Deng, S. T., Lin, Z. T., Tang, H. X., Ullah, S., & Bi, Y. G. (2019). Rapid synthesis of hydroxyapatite nanoparticles via a novel approach in the dual-frequency ultrasonic system for specific biomedical application. Journal of Materials Research, 34, 2796–2806.
Di Mauro, V., Iafisco, M., Salvarani, N., Vacchiano, M., Carullo, P., Ramírez-Rodríguez, G. B., Catalucci, D., et al. (2016). Bioinspired negatively charged calcium phosphate nanocarriers for cardiac delivery of MicroRNAs. Nanomedicine, 11, 891–906.
Sun, L., & Chow, L. C. (2008). Preparation and properties of nano-sized calcium fluoride for dental applications. Dental Materials, 24, 111–116.
Takagi, S., Chow, L. C., Hirayama, S., & Eichmiller, F. C. (2003). Properties of elastomeric calcium phosphate cement–chitosan composites. Dental Materials, 19, 797–804.
Ring, T. A. (1996). Fundamentals of ceramic powder processing and synthesis. Elsevier.
Vogel, G. L., Chow, L. C., & Brown, W. E. (1983). A microanalytical procedure for the determination of calcium, phosphate and fluoride in enamel biopsy samples. Caries Research, 17, 23–31.
Riehemann, K., Schneider, S. W., Luger, T. A., Godin, B., Ferrari, M., & Fuchs, H. (2009). Nanomedicine—Challenge and perspectives. Angewandte Chemie International Edition, 48, 872–897.
Vallet-Regí, M. (2006). Revisiting ceramics for medical applications. Dalton Transactions, 44, 5211–5220.
Reischl, D., & Zimmer, A. (2009). Drug delivery of siRNA therapeutics: Potentials and limits of nanosystems. Nanomed.: Nanotechnol. Biologie et Médecine, 5, 8–20.
Sundriyal, S., Sharma, R. K., & Jain, R. (2006). Current advances in antifungal targets and drug development. Current Medicinal Chemistry, 13, 1321–1335.
Jewell, C. M., & Lynn, D. M. (2008). Multilayered polyelectrolyte assemblies as platforms for the delivery of DNA and other nucleic acid-based therapeutics. Advanced Drug Delivery Reviews, 60, 979–999.
Hu, L., Mao, Z., & Gao, C. (2009). Colloidal particles for cellular uptake and delivery. Journal of Materials Chemistry, 19, 3108–3115.
Graham, F. L., & Van Der Eb, A. J. (1973). A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology, 52, 456–467.
Gonzalez-McQuire, R., Green, D. W., Partridge, K. A., Oreffo, R. O., Mann, S., & Davis, S. A. (2007). Coating of human mesenchymal cells in 3D culture with bioinorganic nanoparticles promotes osteoblastic differentiation and gene transfection. Advanced Materials, 19, 2236–2240.
Xu, Z. P., Zeng, Q. H., Lu, G. Q., & Yu, A. B. (2006). Inorganic nanoparticles as carriers for efficient cellular delivery. Chemical Engineering Science, 61, 1027–1040.
Cai, Y., & Tang, R. (2008). Calcium phosphate nanoparticles in biomineralization and biomaterials. Journal of Materials Chemistry, 18, 3775–3787.
Kakizawa, Y., Furukawa, S., Ishii, A., & Kataoka, K. (2006). Organic–inorganic hybrid-nanocarrier of siRNA constructing through the self-assembly of calcium phosphate and PEG-based block aniomer. Journal of Controlled Release, 111, 368–370.
Green, D. W., Mann, S., & Oreffo, R. O. (2006). Mineralized polysaccharide capsules as biomimetic microenvironments for cell, gene and growth factor delivery in tissue engineering. Soft Matter, 2, 732–737.
Sokolova, V., Kovtun, A., Heumann, R., & Epple, M. (2007). Tracking the pathway of calcium phosphate/DNA nanoparticles during cell transfection by incorporation of red-fluorescing tetramethylrhodamine isothiocyanate–bovine serum albumin into these nanoparticles. JBIC Journal of Biological Inorganic Chemistry, 12, 174–179.
Kurreck, J. (2009). RNA interference: from basic research to therapeutic applications. Angewandte Chemie - International Edition, 48, 1378–1398.
Fire, A. Z. (2007). Gene silencing by double-stranded RNA (Nobel lecture). Angewandte Chemie International Edition, 46, 6966–6984.
Mello, C. C. (2007). Return to the RNAi world: Rethinking gene expression and evolution (Nobel Lecture). Angewandte Chemie International Edition, 46, 6985–6994.
Brummelkamp, T. R., Bernards, R., & Agami, R. (2002). Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell, 2, 243–247.
Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K., & Tuschl, T. (2001). Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498.
Krishnamachari, Y., & Salem, A. K. (2009). Innovative strategies for co-delivering antigens and CpG oligonucleotides. Advanced Drug Delivery Reviews, 61, 205–217.
Cui, Z., & Mumper, R. J. (2003). Microparticles and nanoparticles as delivery systems for DNA vaccines. Critical Reviews in Therapeutic Drug Carrier Systems, 20, 2–3.
Sokolova, V., Kovtun, A., Prymak, O., Meyer-Zaika, W., Kubareva, E. A., Romanova, E. A., ... & Epple, M. (2007). Functionalisation of calcium phosphate nanoparticles by oligonucleotides and their application for gene silencing. Journal of Materials Chemistry, 17:721–727.
Liong, M., Lu, J., Kovochich, M., Xia, T., Ruehm, S. G., Nel, A. E., Zink, J. I., et al. (2008). Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano, 2, 889–896.
Gil, P. R., & Parak, W. J. (2008). Composite nanoparticles take aim at cancer. ACS Nano, 2, 2200–2205.
Shubayev, V. I., Pisanic, T. R., II., & Jin, S. (2009). Magnetic nanoparticles for theragnostics. Advanced Drug Delivery Reviews, 61, 467–477.
Yuan, F. (1998). July) (pp. 164–175). Transvascular drug delivery in solid tumors. In Seminars in radiation oncology.
Ewence, A. E., Bootman, M., Roderick, H. L., Skepper, J. N., McCarthy, G., Epple, M., Proudfoot, D., et al. (2008). Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: A potential mechanism in atherosclerotic plaque destabilization. Circulation Research, 103, 28–34.
Mondéjar, S. P., Kovtun, A., & Epple, M. (2007). Lanthanide-doped calcium phosphate nanoparticles with high internal crystallinity and with a shell of DNA as fluorescent probes in cell experiments. Journal of Materials Chemistry, 17, 4153–4159.
Ramachandran, R., Paul, W., & Sharma, C. P. (2009). Synthesis and characterization of PEGylated calcium phosphate nanoparticles for oral insulin delivery. Journal Biomedical Materials. Research B, 88, 41–48.
Kester, M., Heakal, Y., Fox, T., Sharma, A., Robertson, G. P., Morgan, T. T., Adair, J. H., et al. (2008). Calcium phosphate nanocomposite particles for in vitro imaging and encapsulated chemotherapeutic drug delivery to cancer cells. Nano Letters, 8, 4116–4121.
Liu, T., Tang, A., Zhang, G., Chen, Y., Zhang, J., Peng, S., & Cai, Z. (2005). Calcium phosphate nanoparticles as a novel nonviral vector for efficient transfection of DNA in cancer gene therapy. Cancer Biotherapy & Radiopharmaceuticals, 20, 141–149.
Djelal, B., & áGeorge Truscott, T. (1999). Journal of the Chemical Society. Perkin Transactions, 2, 325–328.
Konan, Y. N., Gurny, R., & Allémann, E. (2002). State of the art in the delivery of photosensitizers for photodynamic therapy. Journal of Photochemistry and Photobiology B: Biology, 66, 89–106.
Urch, H., Vallet-Regi, M., Ruiz, L., Gonzalez-Calbet, J. M., & Epple, M. (2009). Calcium phosphate nanoparticles with adjustable dispersability and crystallinity. Journal of Materials Chemistry, 19, 2166–2171.
Bose, S., Dasgupta, S., Tarafder, S., & Bandyopadhyay, A. (2010). Microwave-processed nanocrystalline hydroxyapatite: Simultaneous enhancement of mechanical and biological properties. Acta Biomaterialia, 6, 3782–3790.
Schwiertz, J., Meyer-Zaika, W., Ruiz-Gonzalez, L., González-Calbet, J. M., Vallet-Regi, M., Epple, M., et al. (2008). Calcium phosphate nanoparticles as templates for nanocapsules prepared by the layer-by-layer technique. Journal of Materials Chemistry, 18, 3831–3834.
Mitchell, L. (1992). Decalcification during orthodontic treatment with fixed appliances—An overview. British J. Orthod, 19, 199–205.
O’reilly, M. M., & Featherstone, J. D. B. (1987). Demineralization and remineralization around orthodontic appliances: An in vivo study. Am J Orthod Dentofacial Orthop, 92, 33–40.
Anuwongnukroh, N., Dechkunakorn, S., & Kanpiputana, R. (2017). Oral hygiene behavior during fixed orthodontic treatment. Dentistry, 7, 1–5.
Ratcliff, P. A., & Johnson, P. W. (1999). The relationship between oral malodor, gingivitis, and periodontitis. A Review Journal Periodontol, 70, 485–489.
Khurshid, Z., Naseem, M., Zafar, M. S., Najeeb, S., & Zohaib, S. (2017). Propolis: A natural biomaterial for dental and oral healthcare. Journal Dentistry Research Dentistry Clinical Dentistry Prospects, 11, 265.
Anderson, G. B., Bowden, J., Morrison, E. C., & Caffesse, R. G. (1997). Clinical effects of chlorhexidine mouthwashes on patients undergoing orthodontic treatment. Americal Journal Orthodontics Dentofacial Orthopedic, 111, 606–612.
Rinaudo, M. (2006). Chitin and chitosan: Properties and applications. Progress Polymer, 31, 603–632.
Costa, E. M., Silva, S., Costa, M. R., Pereira, M., Campos, D. A., Odila, J., ... & Pintado, M. M. (2014). Chitosan mouthwash: Toxicity and in vivo validation. Carbohydrate Polymers, 111:385–392.
Pepla, E., Besharat, L. K., Palaia, G., Tenore, G., & Migliau, G. (2014). Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Annali di Stomatologia, 5, 108.
Allaker, R. P. (2010). The use of nanoparticles to control oral biofilm formation. Journal of Dental Research, 89, 1175–1186.
Jabri, M., Mejdoubi, E., El Gadi, M., & Hammouti, B. (2013). Synthesis and optimization of a new calcium phosphate ceramic using a design of experiments. Research on Chemical Intermediates, 39, 659–669.
Albinali, K. E., Zagho, M. M., Deng, Y., & Elzatahry, A. A. (2019). A perspective on magnetic core–shell carriers for responsive and targeted drug delivery systems. International Journal of Nanomedicine, 14, 1707.
Roggers, R., Kanvinde, S., Boonsith, S., & Oupický, D. (2014). The practicality of mesoporous silica nanoparticles as drug delivery devices and progress toward this goal. An Official Journal of the American Association of Pharmaceutical Scientists, 15, 1163–1171.
Singh, P., Pandit, S., Mokkapati, V. R. S. S., Garg, A., Ravikumar, V., & Mijakovic, I. (2018). Gold nanoparticles in diagnostics and therapeutics for human cancer. International Journal of Molecular Sciences, 19, 1979.
Ramachandran, R., Paul, W., & Sharma, C. P. (2009). Synthesis and characterization of PEGylated calcium phosphate nanoparticles for oral insulin delivery. J. Biomedical Materials Research Part B Applied. Biomaterials, 88, 41–48.
Levingstone, T. J., Herbaj, S., Redmond, J., McCarthy, H. O., & Dunne, N. J. (2020). Calcium phosphate nanoparticles-based systems for RNAi delivery: Applications in bone tissue regeneration. Nanomater, 10, 146.
Dorozhkin, S. V. (2011). Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter, 1, 121–164.
Epple, M. (2018). Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomaterialia, 77, 1–14.
Colilla, M., & Vallet-Regí, M. (2020). Targeted stimuli-responsive mesoporous silica nanoparticles for bacterial infection treatment. International Journal of Molecular Sciences, 21, 8605.
Perez, R. A., Kim, H. W., & Ginebra, M. P. (2012). Polymeric additives to enhance the functional properties of calcium phosphate cements. Journal Tissue Engineering, 3, 1.
Hesaraki, S., Borhan, S., Zamanian, A., & Hafezi-Ardakani, M. (2013). Rheological properties and Injectability of β-Tricalcium phosphate-hyaluronic acid/polyethylene glycol composites used for the treatment of Vesicouretheral reflux. Biomedical Engineering Research, 1, 40–44.
Ruhe, P. Q., Hedberg, E. L., Padron, N. T., Spauwen, P. H., Jansen, J. A., & Mikos, A. G. (2003). rhBMP-2 release from injectable poly (DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. The Journal of Bone and Joint Surgery, 85, 75–81.
Zhao, L., Weir, M. D., & Xu, H. H. (2010). An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials, 31, 6502–6510.
Koempel, J. A., Patt, B. S., O’Grady, K., Wozney, J., & Toriumi, D. M. (1998). The effect of recombinant human bone morphogenetic protein-2 on the integration of porous hydroxyapatite implants with bone. Journal of Biomedical Materials Research, 41, 359–363.
Kaygili, O., Keser, S., Tankut, A. T. E. S., Kirbag, S., & Yakuphanoglu, F. (2016). Dielectric properties of calcium phosphate ceramics. Materials Science, 22, 65–69.
Silva, C. C., Graça, M. P. F., Sombra, A. S. B., & Valente, M. A. (2009). Structural and electrical study of calcium phosphate obtained by a microwave radiation assisted procedure. Physical B: Condensed Matter, 404, 1503–1508.
Lyczko, N., Nzihou, A., & Sharrok, P. (2014). Calcium phosphate sorbent for environmental application. Procedia Engineering, 83, 423–431.
Lemlikchi, W., Sharrock, P., Mecherri, M. O., Fiallo, M., & Nzihou, A. (2012). Treatment of textile waste waters by hydroxyapatite co-precipitation with adsorbent regeneration and reuse. Waste Biomass Valorization, 3, 75–79.
Salama, A. (2019). Cellulose/calcium phosphate hybrids: New materials for biomedical and environmental applications. International Journal of Biological Macromolecules, 127, 606–617.
Acknowledgements
The authorsgratefully acknowledge the National Institute of Technology (NIT), Arunachal Pradesh, India, for the assistance and support.
Funding
This study received financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi, India (project grant no. 22(0847)/20/EMR-II, dated: 10.12.2020).
Author information
Authors and Affiliations
Contributions
Conception: Nabakumar Pramanik.
Literature survey: Abinash Das, and Togam Ringu.
Images and table design: Abinash Das, and Togam Ringu.
Manuscript composition and referencing: Abinash Das, Togam Ringu, Sampad Ghosh, and Nabakumar Pramanik.
Manuscript moderation and design: Abinash Das, Sampad Ghosh, and Nabakumar Pramanik.
Edit and correction: Sampad Ghosh and Nabakumar Pramanik.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, A., Ghosh, S., Ringu, T. et al. A Focus on Biomaterials Based on Calcium Phosphate Nanoparticles: an Indispensable Tool for Emerging Biomedical Applications. BioNanoSci. 13, 795–818 (2023). https://doi.org/10.1007/s12668-023-01081-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01081-6