Abstract
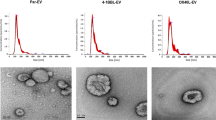
Nowadays, immunotherapy, in particular immunotherapeutic vaccines, is a promising approach in the treatment of cancer which has already demonstrated its effectiveness. Extracellular vesicles (EVs), which are capable of delivering biologically active agents to the cells, can be a promising candidate for such vaccines. M14 human melanoma cells were transduced with lentivirus encoding interleukin (IL)–2. Membrane vesicles from M14 cells expressing IL-2 were isolated using cytochalasin B. The interaction of membrane vesicles with human peripheral blood mononuclear cells has been analyzed. Activation of T cells was shown, as well as a decrease in the number of NK cells, after cultivation with tumor-derived vesicles. However, no cytotoxic activity of T cells after cultivation with tumor-derived vesicles was observed, which can be explained by their immunosuppressive properties. On the other hand, such vesicles can be a promising source of tumor-specific antigens for dendritic vaccines. Therefore, further studies are required in view of the possible use of tumor-derived vesicles as a target antigen for dendritic cells.




Similar content being viewed by others
Data Availability
The authors confirm that all relevant data used to support the findings of this study are included within the article.
References
Shupp, A. B., Kolb, A. D., & Bussard, K. M. (2020). Novel techniques to study the bone-tumor microenvironment. Advances in Experimental Medicine and Biology, 1225, 1–18.
Chulpanova, D. S., et al. (2018). Therapeutic prospects of extracellular vesicles in cancer treatment. Frontiers in Immunology, 9, 1534.
Mathieu, M., et al. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature Cell Biology, 21(1), 9–17.
Kawamoto, T., et al. (2012). Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS ONE, 7(3), e34045.
Wang, Y., et al. (2020). Tumor-derived EV-encapsulated miR-181b-5p induces angiogenesis to foster tumorigenesis and metastasis of ESCC. Mol Ther Nucleic Acids, 20, 421–437.
Andrade, L. N. S., et al. (2019). Extracellular vesicles shedding promotes melanoma growth in response to chemotherapy. Science and Reports, 9(1), 14482.
Robbins, P. D., & Morelli, A. E. (2014). Regulation of immune responses by extracellular vesicles. Nature Reviews Immunology, 14(3), 195–208.
Prada, I., & Meldolesi, J. (2016). Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. International Journal of Molecular Sciences, 17(8), 1296.
Choudhry, H., et al. (2018). Prospects of IL-2 in cancer immunotherapy. BioMed Research International, 2018, 9056173.
Chulpanova, D. S., et al. (2021). Cytochalasin B-induced membrane vesicles from human mesenchymal stem cells overexpressing IL2 are able to stimulate CD8(+) T-killers to kill human triple negative breast cancer cells. Biology (Basel), 10(2), 141.
Davis, L. E., Shalin, S. C., & Tackett, A. J. (2019). Current state of melanoma diagnosis and treatment. Cancer Biology & Therapy, 20(11), 1366–1379.
Overwijk, W. W., Tagliaferri, M. A., & Zalevsky, J. (2021). Engineering IL-2 to give new life to T cell immunotherapy. Annual Review of Medicine, 72, 281–311.
Tarhini, A. A., et al. (2007). Durable complete responses with high-dose bolus interleukin-2 in patients with metastatic melanoma who have experienced progression after biochemotherapy. Journal of Clinical Oncology, 25(25), 3802–3807.
Pol, J. G., et al. (2020). Effects of interleukin-2 in immunostimulation and immunosuppression. Journal of Experimental Medicine, 217(1), e20191247.
Jen, E. Y., et al. (2012). IL-2 regulates the expression of the tumor suppressor IL-24 in melanoma cells. Melanoma Research, 22(1), 19–29.
Wrangle, J. M., et al. (2018). IL-2 and beyond in cancer immunotherapy. Journal of Interferon and Cytokine Research, 38(2), 45–68.
Schwartz, R. N., Stover, L., & Dutcher, J. P. (2002). Managing toxicities of high-dose interleukin-2. Oncology (Williston Park N.Y.), 16(11 Suppl 13), 11–20.
Poindexter, N. J., et al. (2005). Cytokine induction of interleukin-24 in human peripheral blood mononuclear cells. Journal of Leukocyte Biology, 78(3), 745–752.
Wahlgren, J., et al. (2012). Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Research, 40(17), e130.
Zhao, S., et al. (2020). Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. Journal of Hematology & Oncology, 13(1), 156.
Hu, J. L., et al. (2019). CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Molecular Cancer, 18(1), 91.
Zhou, C., et al. (2021). Cancer-secreted exosomal miR-1468-5p promotes tumor immune escape via the immunosuppressive reprogramming of lymphatic vessels. Molecular Therapy, 29(4), 1512–1528.
Sharma, P., et al. (2020). Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells. Science and Reports, 10(1), 92.
Chen, G., et al. (2018). Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature, 560(7718), 382–386.
Li, Q., et al. (2021). Tumor-derived extracellular vesicles: Their role in immune cells and immunotherapy. International Journal of Nanomedicine, 16, 5395–5409.
Zhao, J., et al. (2019). Tumor-derived extracellular vesicles inhibit natural killer cell function in pancreatic cancer. Cancers (Basel), 11(6), 874.
Funding
This research was funded by the Russian Science Foundation grant no. 22–24-20018 and Kazan Federal University Strategic Academic Leadership Program.
Author information
Authors and Affiliations
Contributions
Conceptualization: F.I.Y., K.K.V., and V.V.S. Methodology: F.I.Y., K.K.V., and V.V.S. Formal analysis: F.I.Y. and K.K.V. Investigation: D.S.C. (flow cytometry analysis) and E.R.A. (confocal microscopy). Writing—original draft preparation: F.I.Y. and K.K.V. Writing—review and editing: V.V.S. and D.S.C. Visualization: K.K.V. Supervision: A.A.R. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Research Involving Human and Animals Statement
An informed consent was obtained from all subjects involved in the study. All manipulations were carried out in accordance with approved ethical standards and current legislation (the protocol was approved by the Committee on Biomedical Ethics of Kazan Federal University (No. 3, 03/23/2017)). This study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Filin, I.Y., Kitaeva, K.V., Chulpanova, D.S. et al. Tumor-Derived Membrane Vesicles from the IL-2 Overexpression Melanoma Cells Affect on the Expression of Surface Markers of Human Peripheral Blood Mononuclear Cells In Vitro. BioNanoSci. 13, 81–87 (2023). https://doi.org/10.1007/s12668-022-01044-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-022-01044-3